Klebsiella pneumoniae Ghosts as Vaccine Using Sponge Like Reduced Protocol
Menisy MM, Hussein A, Abd El-Kader Ghazy A, Sheweita S and Amro Abd A Fattah Amara
DOI10.21767/2573-5365.100034
Menisy MM1, Hussein A1, Abd El-Kader Ghazy A2, Sheweita S1 and Amro Abd Al Fattah Amara1,3*
1 Department of Biotechnology, Institute of Graduate Studies and Research, Alexandria University, 163 El-Horreya Avenu, P.O. Box 832, Alexandria 21526, Egypt
2 Department of Immunology, Medicinal Research Institute, Alexandria University, Egypt
3 Department of Protein Research, Genetic Engineering and Biotechnology Research Institute, City for Scientific Research and Technology Applications, New Borg Al-Arab, P.O. Box. 21934, Alexandria, Egypt
- *Corresponding Author:
- Al-Fattah Amara AA
Department of Biotechnology, Institute of Graduate Studies and Research
Department of Protein Research, Genetic Engineering and Biotechnology Research Institute
City for Scientific Research and Technology Applications, Alexandria University
163 El Horreya Avenu, P.O. Box 832, Alexandria 21526, Egypt.
Tel: 203-4593422
E-mail: amroamara@web.de
Received Date: June 08, 2017; Accepted Date: July 26, 2017; Published Date: July 28, 2017
Citation: Menisy MM, Hussein A, Ghazy A AE, Sheweita S, Amara AAAF (2017) Klebsiella pneumoniae Ghosts as Vaccine Using Sponge Like Reduced Protocol. Cell Mol Med. Vol. 3 No. 2:11
Abstract
Recently, different microbial ghosts (BGs) were prepared using a protocol based on critical chemical concentrations. In the original protocol, it was given the name “Sponge Like” (SL) protocol. The protocol was reduced to be more reliable and given a name “Sponge Like Reduced Protocol” (SLRP). In this study, using SLRP we succeeded to prepare Klebsiella pneumoniae (KPGs) with correct 3D structure and surface antigens. The study has included oral, S/C, inhalation, I/P and I/M vaccination with KPGs and controlled with challenge test. Results of both the SLRP and the animal experiments prove that we have KPGs able to immunize rats subjected to different routes of administration against viable K. pneumoniae. The evaluation of the immune response, include humoral and cellular immune responses, IFN-γ production, phagocytic activity, NBT reduction activity, serum agglutination titer and bacterial load of various tissues, was performed in BGvaccinated animals subsequently challenged with K. pneumoniae. The results were compared with animals that were not immunized with the KPGs. KPGs not only promoted the generation of high titer antibodies and IFN-γ production but also stimulated a significant increase in phagocytic activity and NBT reduction and a marked decrease in bacterial load of various tissues, indicated that the vaccine was able to induce clearance of intracellular K. pneumoniae. The protective effects of BG vaccination in rats against virulent K. pneumoniae were a result of the induction of a more effective immune response in vaccinated animals than that observed with the non-vaccinated animals. These findings demonstrate the potential of K. pneumoniae ghosts to be used as effective vaccines.
https://maviyolculuk.online/
https://mavitur.online/
https://marmaristeknekirala.com.tr
https://tekneturumarmaris.com.tr
https://bodrumteknekirala.com.tr
https://gocekteknekirala.com.tr
https://fethiyeteknekirala.com.tr
Keywords
Microbial ghosts; Bacteriophage; DNA; Vaccine
Abbrevations
BG: Bacterial Ghosts; BGsQ: BGs Quality; MGC: Minimum Growth Concentration; MIC: Minimum Inhibition Concentration; MIF: Mean Intensity of Fluorescence; OMP: Outer Membrane Proteins; SDS: Sodium Dodecyl Sulfate; Sl: Sponge Like; SLRP: Sponge Like Reduced Protocol; SGPT: Serum Glutamic Pyruvic Transaminase; SGOT: Serum Glutamic Oxaloacetic Transaminase
Introduction
Bacterial ghosts (BGs) are empty non-deformed bacterial envelopes of gram-negative bacteria produced by controlled expression of cloned gene E from bacteriophage, forming a lysis tunnel structure within the envelope of the living bacteria. BGs are devoid of cytoplasmic content and possess all bacterial bioadhesive surface properties in their original state. Using cloned or genetically modified elements (E lysis gene) for BGs preparation could have some risk factors. Genetic elements could by one or other way interact with others that pose a risk when any of them entered into our bodies [1]. For that BGs preparation was recently established using methods other than the E lysis gene, such as using critical concentrations from chemical compounds able to convert viable cells to BGs [2]. Sodium dodecyl sulfate (SDS) and sodium hydroxide (NaOH) were reported for their ability to interfere with the bacterial cell wall. H2O2 is well known for its ability (as an oxidant) to degrade the DNA and the other genetic elements [2]. There is no reliable K. pneumoniae vaccine produced so far, although many attempts were made [3,4].
In 1982 LPS vaccines have a relatively high incidence of side effects, as well as the problem of requiring a mixture of various LPS serotypes to make the vaccine effective in the treatment of K. pneumoniae infections [5,6] used the porin of K. pneumoniae as a protective vaccine in mice [5] induced good protective activity by immunizing mice with capsular polysaccharide of K. pneumoniae [7]. However, this activity was not observed in man. Lee et al. in 2000 reported that outer membrane proteins (OMPs) elicit antibodies with protective efficacy against K. pneumoniae infection in burned patients only [8]. Used heatkilled K. pneumoniae as a vaccine against the infection, but this immunogen caused serious adverse effects [9]. The use of killed pathogens as substitutes for the living infectious agents was widely used as a principle for vaccine development. BGs can be used as vaccine candidates with intrinsic adjuvant properties based on well-known immune-stimulating compounds such as lipopolysaccharides, lipid A, and peptidoglycan, to potentiate non-specifically the immune response to a target antigen [10].
Materials and Methods
Bacterial strain
K. pneumoniae strain was used in this study was kindly provided by Bacteriology Department at the Faculty of Medicine, Tanta University by Prof. Mohamed Zakaria on MacConkey plates from Egyptian patients suffering from pneumonia.
Bacterial cultivation
K. pneumoniae was cultivated in 500 mL NB (in one-liter flask) under shaking condition at 37°C for 72 hrs.
Determination of the MGC minimum growth concentration (MIC) and minimum growth concentration (MGC) for NaOH, SDS, and H2O2
The MIC and MGC for each of NaOH, SDS, and H2O2 were determined using standard criteria [11,12]. 10% of each of NaOH and SDS as well as 30% H2O2 stock solution were prepared. Standard serial dilution method was used to determine MIC and MGC values, where 1 mL of the above solutions were added (each) to 4 mL in 10 mL test tube (to gain the final volume 5 mL and the 1:10 dilution), and so on. 200 μL of an overnight K. pneumoniae culture was added to each tube in the serial dilution experiment. In case of CaCO3 the used amount of +1 value was 1.05 μg/mL, while −1 value was 0.35 μg/mL. The test tubes were then incubated overnight at 37°C under static condition [11].
Sponge like reduced protocol for preparing the BGs
Two experiments with different parameters (Table 1) were conducted. The two experiments were selected from the best results obtained by Amara et al. They are mainly experiments numbers one and eleven in the original SL protocol [2].
| Experiment No. |
NaOH step | SDS+CaCO3 step | H2O2 Step | Ethanol step | (BGQ)% | ||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | DNA | Protein | DNA | Protein | DNA | Protein | DNA | ||
| µg ml-1 | µg ml-1 | µg ml-1 | µg ml-1 | µg ml-1 | µg ml-1 | µg ml-1 | µg ml-1 | ||
| 1 | 1425 | 186.35 | 171.7 | 101.3 | 89.5 | 33.25 | 22.61 | 19.45 | 100 |
Table 1: The amount of the DNA and Protein realized during different ghost’s preparation steps.
Determination of the DNA and protein concentrations
The concentrations of the DNA and the protein were derived from the spectrophotometer measurement (Thermo Scientific-VISIONpro Software V4.30) and determined according to Amara et al.
KPGs evaluation using light microscope (Optika)
Bacterial smears for both preparations were stained using crystal violet for 30 seconds. The cells were examined using the light microscope. The BGs quality (BGsQ) was determined for each preparation based on the quality of the 3D structure as either being correct or deformed. The overall BGsQ for each was given as a %.
Transmission electron microscope for examination of K. pneumoniae Gs
Transmission electron microscope (JEOL TEM 100 CX) was used for the examination of K. pneumoniae Gs.
Viability test
The prepared KPGs were evaluated for the existence of any still viable cells, where samples were taken from each preparation and grown in MacConkey agar plates. The plates then were incubated at 37°C for two days.
Animals and experimental design
Fifty-two male Sprague-Dawley rats weighing 110 ± 10 g were purchased from the animal house of the Institute of Graduate Studies and Research, Alexandria University, Egypt. After two weeks of acclimatization, rats were randomly assigned to six groups. The tested substances for the animals according to the following experimental protocol: groups for this experiment, A: control group included 13 rats, which not inoculated with bacterial ghost and five residual groups included B: 5 rats I/M, C: 5 rats I/P, D: 5 rats inhalation, E: 12 rats oral, F: 12 rats S/C which inoculated with bacterial ghost each received 100 μl (107 CFU) of KPGs in sterile saline. Initial dose of KPGs were administered at week 1 and followed by two booster doses at week 3 and week 5, on week 6. All rats were challenged with 100 μl (108) CFU of virulent K. pneumoniae strain at weak 6.
Challenge test
At the end of 4th week, each group was challenged I/P, inhalation and vagina with 100 μl sterile saline containing (108) pathogenic strain of K. pneumoniae. The clinical signs and mortalities were monitored for 7 days post challenge. After one week from the 2nd booster, 12 animals (8 oral, 7 S/C) had received 108 CFU of live enteropathogenic K. pneumoniae (1st challenge) I/P, inhalation and vaginal one dose for I/P group and three doses for vaginal and inhalation groups on three successive days. After one week from the 3rd booster, 18 animals (5 control, 3 oral, 2 S/C, 2 I/M, 2 I/P, 1 inhalation, 1 I/P+Adj., 1 oral+Adj., 1 inhalation+Adj.) had received 108 CFU of live enteropathogenic K. pneumoniae (final challenge) I/P, inhalation and vaginal three doses on three successive days.
Phagocytic activity
Phagocytic activity was investigated using living K. pneumoniae and KPGs, determined as the percentage of phagocytizing neutrophils (one or more bacteria ingestion) and as the mean intensity of fluorescence (MIF) value, which equaled the mean number of bacteria phagocytized by the cells. Cells were visualized using light microscope "LM"and immuno-fluorescent microscope "IFM" with the aid of acridine orange due to determine living or killed bacteria.
IFN-γ level
IFN-γ levels in different treatments were measured according to manufacturers, instructions using Rat IFN-γ platinum ELISA (Ready- to -use Sandwich ELISA, eBioscience, North America).
Slide agglutination test for KPGs
Antibody titers were measured by slide agglutination titer. Glass slide was cleaned well, wiped free of water. One drop of each sample was placed with antiserum of KPGs vaccinated animals. The content of each circle was mixed using separate wooden applicator or stick and spread to fill the whole area of individual circle. The slide was shacked for one to three minutes and observed for agglutination [13].
Nitroblue Tetrazolium Test (NBT)
Heparinized blood samples are incubated with a buffered solution of NBT under carefully controlled conditions [14]. Smears are then prepared, stained and examined microscopically to determine the percentage of neutrophils showing intracytoplasmic deposits of reduced NBT insoluble formazan).
Isolation of LPS
Lipopolysaccharide (LPS) was extracted from K. pneumoniae cells, cultured overnight on NB medium, by the phenol–water method of Westphal and Jann in 1965. Preparations were purified by the treatment with 2% cetavlon before being dialyzed extensively against distilled water and then lyophilized.
SDS-PAGE analysis
SDS-PAGE was performed on 12% (w/v) polyacrylamide gels as previously described [15]. Lanes were loaded with either freshly grown bacteria or KPGs (10 ml or 1 mg, respectively, each corresponding to (108 CFU) in saline or LPS (300 ng) preparations. All samples were solubilized in sample buffer containing 2% (w/v) SDS and 5% (w/v) β-mercaptoethanol and heated at 100°C for 3 min prior to loading. Gels were electrophoresed at 100 V through the stacking gel and then at 150 V for about 3 hrs. Molecular weight markers (Pharmacia) following electrophoresis, LPS gels were subjected to Ammonia Silver staining using the Phast GelTH (Pharmacia) kit. Other gels were fixed and stained with Coomassie blue R250 (Sigma).
Bacteriological analysis for measurement of bacterial load in tissues
Infected rats Lungs, liver, spleen, gut and vagina were harvested and equal wet tissue weights were homogenized and briefly centrifuged to remove gross particulate matter. Serial ten-fold dilutions of tissue homogenates were applied to selective agar plates and incubated at 37°C for 24 hrs. under aerobic conditions. Plates were subsequently analyzed by colony counts and expressed as CFU/g tissue weight [16].
Statistical analysis of the data
All data is expressed as mean + standard error of the mean using IBM SPSS software version 20.0. [17]. For normally distributed data, comparison between more than two independent populations was done using F-test (ANOVA) to be used and Post Hoc test (LSD). Significance of the obtained results was judged at the 5% level.
Results and Discussion
Recently a new protocol for BGs preparation was introduced. The protocol is able to evacuate different microbes including gram positive, negative, spore former, capsulated etc., bacteria. Even it is succeeded to prepare ghosts from eukaryotic cells as well as viruses. The protocol find acceptance worldwide particularly due to its reliability, cost effective and applicability with different microbes. The main point start by determining the MIC and the MGC of the used chemical compounds. In case of NaOH; the MIC and the MGC were 0.8 mg/ml and 0.08 mg/ml, respectively. In case of SDS; the MIC and the MGC were 0.1 mg/ml and 0.01 mg/ ml, respectively as in E. coli. In case of H2O2; the MIC and the MGC were 12 mg/ml and 2.4 mg/ml, respectively. In case of CaCO3; the used amount of +1 value was 1.05 μg/ml while -1 values were 0.35 μg/ml according to Amara et al. in 2013.
The results show correct release of both of DNA and protein during the preparation steps. The amount of the protein is greater than that of DNA (Table 1). That is logic, particularly at the H2O2 step. None of the cells upon cultivation on NA medium for two days at 37°C has shown any growth.
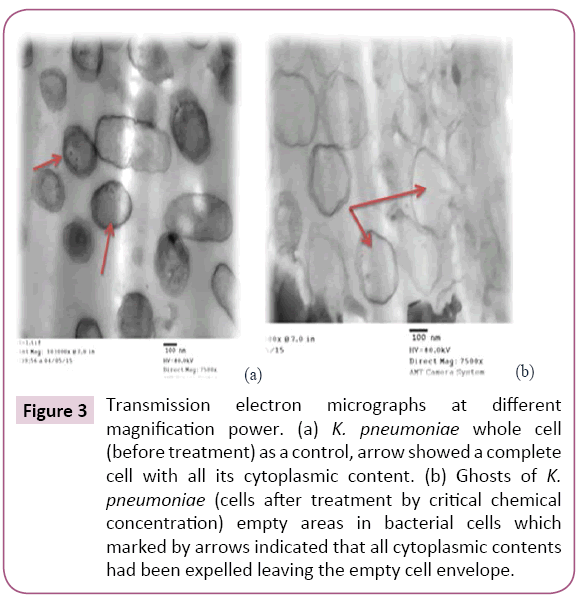
Chemically induced KPGs were produced for the first time by critical chemical concentration method as described by Amara et al. In the present study MIC determination is critical step in the BGs preparation. For that and by using several steps in the protocol the bacterial cell loss their content of DNA and protein. The first point is to expose the bacterial cells to the chemical compounds either alone or in correct combination (did not enable chemical interactions), and the second point is to enable several centrifugations and washing steps to remove the DNA and the protein content completely. The gentle centrifugation enables fine pressing to the cells to enable them to get rid of their cytoplasm constituents. The quality of the prepared BGs (QBGs) was monitored using the light, the fluorescent and the electron microscope. The correct 3D structure of the cells is presented in Figures 1-3. Treated bacterial ghost cells and non-treated control cells were subjected to analyze remaining protein by SDS-PAGE analysis. Non-treated control cells showed very clear strong protein bands. This electrophoretic profile indicates that the protein extract contains several antigenic proteins from various parts of the bacterial cell, as expected. While the chemically treated cells show weaker protein bands. This is due to the lack of cytoplasmic contents in BGs cells [18]. In addition, the complete degradation of bacterial genomic DNA was determined using agarose gel electrophoresis (Figure 4). This result indicates that DNA free KPGs are successfully induced by critical chemical concentration of NaOH, SDS and H2O2. Also, Amara et al. in 2013a showed similar results that the genomic DNA of E. coli JM 109 bacterial ghost was completely degraded using sponge like reduced protocol of critical chemical compounds.
Figure 3: Transmission electron micrographs at different magnification power. (a) K. pneumoniae whole cell (before treatment) as a control, arrow showed a complete cell with all its cytoplasmic content. (b) Ghosts of K. pneumoniae (cells after treatment by critical chemical concentration) empty areas in bacterial cells which marked by arrows indicated that all cytoplasmic contents had been expelled leaving the empty cell envelope.
Serum Agglutination titer is highly positive in all vaccinated groups when compared to control groups (Figure 5). This indicates that KPGs vaccine stimulate humoral immune response. After challenge, there was marked decrease in agglutination titer in vaccinated group when compared to an increase in agglutination titer in control group. This is due to consumption of specific Abs in neutralization of K. pneumoniae used in the challenge, thus mild positive reaction occurs, so our vaccine gives long lasting effect. Control group gives highly positive reaction due to first exposure and absence of specific Abs in serum. We noticed marked increase in agglutination titer after each booster due to secondary humoral immune response against each booster that leads to increase amount of specific Abs.
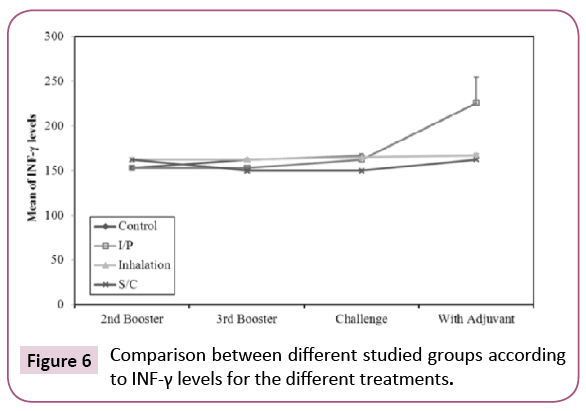
The concentrations of IFN- γ in the different groups are summarized in Figure 6. After booster with K. pneumoniae ghost (KPGs), there was statistically significant increase (p ≤ 0.05) in INF-γ levels in groups vaccinated by inhalation and S/C routes when compared to the control group. This indicates a significant activation of TH cells; So KPG is able to activate the specific immunity in vaccinated rats. INF-γ levels were high in inhalation and S/C vaccinated rats, there was significant increase in control group after challenge when compared to before challenge, in the same time there was insignificant decrease in S/C vaccinated rats after challenge when compared to before challenge. This may be due to neutralization of K. pneumoniae used in the challenge by specific Abs in the sera of vaccinated rats so mild activation of TH cells occurs. This indicates that KPG is able to induce specific humoral immune response among vaccinated rats especially when given subcutaneously.
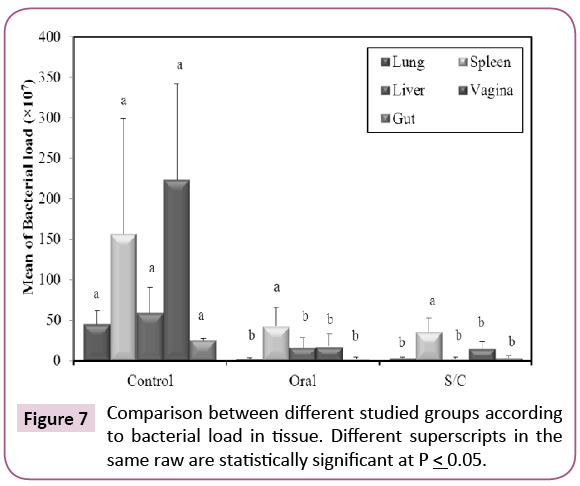
Our results revealed that the bacterial load from the internal organs was a significantly reduced in all vaccinated groups when compared with control non-vaccinated group (p ≤ 0.05) (Figure 7), but is still high. This may be due to lower efficacy of dose used, either initial or booster dose, so we recommend increasing initial and two booster doses to overcome bacterial load. Rats from S/C group show higher protection than orally vaccinated group. So, efficacy of KPGs is more prominent when given subcutaneous. Some studies on BG vaccines have reported that BG-vaccinated animals not only exhibit a good immune response but are also resistant to infection by highly virulent strains, indicating the protective effect of the BG vaccine [19-22]. Results of bacteriological examination indicate that the chemically induced KPGs are an effective vaccine candidate against virulent K. pneumoniae challenge.
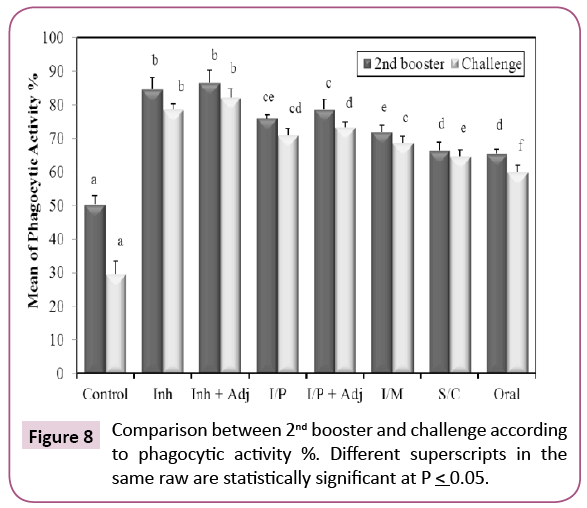
Our results showed that KPGs are potent antigens causing activation of phagocytic cells and stimulation of non-specific innate immunity that results in rapid elimination of the bacteria and presentation of foreign antigen to T and B lymphocytes to start the specific, long lasting immune response [23]. All groups showed significant increase when compared with control (Figure 8). The percent of active phagocytic cells was the highest increase after the second booster in (inhalation+adjuvant) group. Freund’s adjuvant was used (1800 μl saline+750 μl concentrated Freund’s adjuvant). This indicated that the antibodies exhibited a strong opsonization effect. The phagocytic process is the principal mechanism for destruction of extracellular bacterial pathogens in mammals [24].
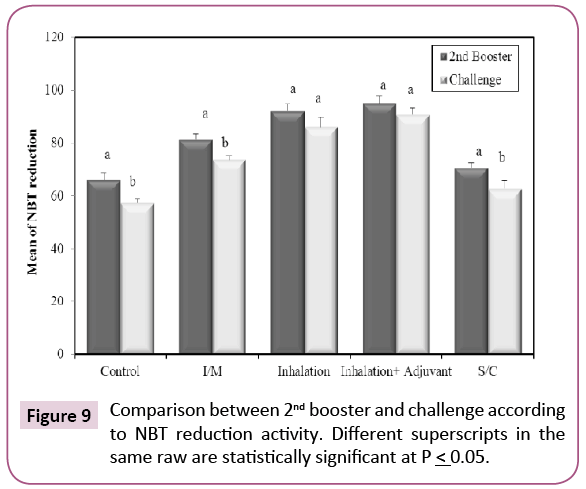
After second booster schedule, there was a significant increase in the percentage of positive NBT neutrophils (Figure 9) in all the vaccinated groups when compared with control group (p ≤ 0.05). After challenge, there was a significant increase in number of positive cells in all vaccinated groups when compared with non-vaccinated group (control) (p ≤ 0.05). This indicates that KPGs caused increase in the killing activity of phagocytic cells especially when given by subcutaneous rout. The NBT test shows that numbers of neutrophils are influenced by the vaccination with KPGs. Many recent experiments have shown that the immunostimulants can be given alone to induce the in vitro and in vivo responses [25]. It is well known that these agents stimulate the non-specific immune response (also called innate or natural protection) and boost the specific immune response. Therefore, immune-stimulants can also be used as adjuvants, to heighten the specific immune response. We can conclude that according to the results of the present study; the NBT is a useful tool for the follow up of the stimulating effects KPGs as a vaccine.
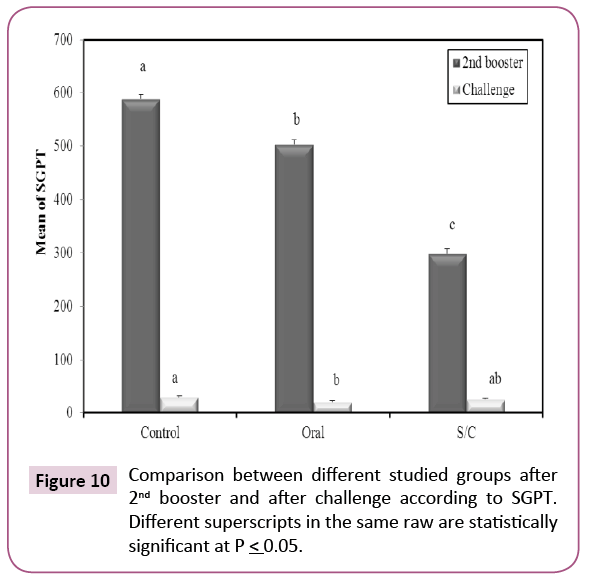
After second booster; there was significant decrease in serum glutamic oxaloacetic transaminase (SGPT) among immunized groups when compared with control group (Figure 10). In serum, glutamic oxaloacetic transaminase (SGOT) there was significant decrease in oral group when compared with control and S/C (p ≤ 0.05) (Figure 11). These results indicate that KPGs as a vaccine provided complete protection for liver cells against highly virulent K. pneumoniae strain.
Conclusion
In conclusion, our study concern with developing KPGs using the SLRP. that the first step was by determining both of the MIC and MGC of (NaOH), (H2O2), (SDS) and (CaCO3). By following the SLRP we succeeded to prepare KPGs with correct 3D structure and surface antigen, empty and dead. KPGs could contribute in the vaccine-induced protection against viable K. pneumoniae infection. The obtained results prove the role of humoral and cell-mediated immune responses in KPGs vaccinated rats upon infection with K. pneumoniae. The BG vaccine not only induced powerful humoral and cell-mediated immune responses in vaccinated rats, but it also provided complete protection against highly virulent K. pneumoniae. Our findings have a promising implication for future Klebsiella vaccine research. The present study, demonstrated that subcutaneous immunization of KPGs can induce strong immune responses which leads to a significant
References
- Makino K, Yokoyama K, Kubota Y, Yutsudo CH, Kimura S, et al. (1999) Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157: H7 derived from the Sakai outbreak. Genes Genet Syst 74: 227-239.
- Amara AA, Salem-Bekhit MM, Alanazi FK (2013) Sponge-like: A new protocol for preparing bacterial ghosts. ScientificWorldJournal2013:545741.
- Cryz S, Mortimer P, Mansfield V, Germanier R (1986) Seroepidemiology of Klebsiellabacteremic isolates and implications for vaccine development. J ClinMicrobiol 23: 687-690.
- Fung CP, Hu BS, Chang FY, Lee SC, Kuo BI, et al. (2000) A 5-year study of the seroepidemiology of Klebsiella pneumoniae: High prevalence of capsular serotype K1 in Taiwan and implication for vaccine efficacy. J Infect Dis 181: 2075-2079.
- Gilleland H, Parker M, Matthews J, Berg R (1984) Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun44: 49-54.
- Tomás JM, Camprubi S, Merino S, Davey M, Williams P (1991) Surface exposure of 01 serotype lipopolysaccharides in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun 59: 2006-2011.
- Pier G, Sidberry H, Zolyomi S, Sadoff J (1978) Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa.Infect Immun 22: 908-918.
- Lee LNG, Jung SB, Ahn BY, Kim YH, Kim JJ, et al. (2000) Immunization of burn-patients with a Pseudomonas aeruginosa outer membrane protein vaccine elicits antibodies with protective efficacy. Vaccine 18: 1952-1961.
- Baumann U (2008) Mucosal vaccination against bacterial respiratory infections. Expert Rev Vaccines 7: 1257-1276.
- Szostak MP, Hensel A, Eko FO, Klein R, Auer T, et al. (1996) Bacterial ghosts: Non-living candidate vaccines. JBiotechnol 44: 161-170.
- Andrews JM (2001) Determination of minimum inhibitory concentrations. JAntimicrobChemother 48: 5-16.
- Amara AA, Salem-Bekhit MM, Alanazi FK (2013) Preparation of bacterial ghosts for E. coli JM 109 using: Sponge-like reduced protocol. Asian J Biol Sci 6: 363.
- Cole JR, Sulzer CR, Pursell AR (1973) Improved microtechniquefor the leptospiral microscopic agglutination test. ApplMicrobiol 25: 976-980.
- Czarnetzki BM, Belcher RW (1974) The nitroblue tetrazolium test in dermatologic patients. Arch Dermatol 109: 36-39.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685.
- Buras JA, Rice L, Orlow D, Pavlides S, Reenstra WR, et al. (2004) Inhibition of C5 or absence of C6 protects from sepsis mortality. Immunobiology 209: 629-635.
- Kirkpatrick L, Feeney B (2012) A simple guide to IBM SPSS: For version 20.0: Cengage Learning.
- Vinod N, Oh S, Kim S, Choi CW, Kim SC, et al. (2014) Chemically induced salmonella enteritidis ghosts as a novel vaccine candidate against virulent challenge in a rat model. Vaccine 32:3249-3255.
- Jalava K, Hensel A, Szostak M, Resch S, Lubitz W (2002) Bacterial ghosts as vaccine candidates for veterinary applications. J Control Release 85: 17-25.
- Panthel K, Jechlinger W, Matis A, Rohde M, Szostak M, et al. (2003) Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infect Immun 71: 109-116.
- Mayr UB, Haller C, Haidinger W, Atrasheuskaya A, Bukin E, et al. (2005) Bacterial ghosts as an oral vaccine: A single dose of Escherichia coliO157: H7 bacterial ghosts protects mice against lethal challenge. Infect Immun 73: 4810-4817.
- Hensel A, Huter V, Katinger A, Raza P, Strnistschie C, et al. (2000) Intramuscular immunization with genetically inactivated (ghosts) Actinobacilluspleuropneumoniae serotype 9 protects pigs against homologous aerosol challenge and prevents carrier state. Vaccine 18: 2945-2955.
- Abbas AK, Lichtman AH, Pillai S (2014) Cellular and molecular immunology: with STUDENT CONSULT Online Access: Elsevier Health Sciences, Netherlands.
- Cohn ZA (1978) The activation of mononuclear phagocytes: Fact, fancy, and future. J Immunol 121: 813-816.
- Anderson DP (2004) Immunostimulants, vaccines, and environmental stressors in aquaculture: NBT assays to show neutrophil activity by these immunomodulators. AvancesenNutriciónAcuícola VII. Mem VII Simp Intern NutricAcuí, Sonora/México, USA 16.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences