Laser Ablation Inductively Coupled Plasma Mass Spectrometry in Biomedicine and Clinical Diagnosis
Ralf Weiskirchen and Ricarda Uerlings
DOI10.21767/2573-5365.100003
Ralf Weiskirchen* and Ricarda Uerlings
Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry, RWTH University Hospital Aachen, Aachen, Germany
- *Corresponding Author:
- Ralf Weiskirchen
Institute of Molecular Pathobiochemistry, Experimental Gene Therapy and Clinical Chemistry (IFMPEGKC), RWTH University Hospital Aachen, Aachen, Germany.
Tel: +49-(0)241-8088683
Fax: +49-(0)241-803388683
E-mail: rweiskirchen@ukaachen.de
Received date: October 10, 2015; Accepted date: October 28, 2015; Published date: November 02, 2015
Abstract
Background: Toxic metal accumulation in the body that may be caused by inherited disorders, chronic intake, or acute intoxication. Vice versa, inadequate daily supply with a specific trace element results in shortcomings and manifests in clinical symptoms. In diagnostics, the determination of exact metal concentrations or the measurement of proteins correlating with the concentration of a specific metal is widespread. In addition, several histopathological stains are available, allowing identification of specific metal deposits in tissue. The introduction of novel metal imaging techniques has extended the repertoire of analytical possibilities. In particular, laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) has undergone an intensive progress in recent years. It has multi-element capability, granting the analysis of a large variety of biological materials with high spatial resolution. It further can identify and quantitate a large number of metals and metalloids at extremely low concentrations. Methods and findings: We here optimized and extended previous studies in which we used LA-ICP-MS for trace metal imaging in livers and brains of wild type and Atp7b deficient mice that represents an experimental model of human Wilson's disease. We show that the observed alterations in metal content and distribution are closely linked to the progression of the disease. While the accumulation of copper in the brain can be assigned to special regions, the overload with copper is initially distributed uniformly, while the regional distribution of individual metals is significant modulated in necrotic and tumorigenic tissue areas.
Conclusion: These illustrations show that LA-ICP-MS is a highly powerful and innovative analytical technique that will have tremendous impact on biomedical research and diagnostics of metal disorders. Abbreviations: Atp7b: gene encoding a copper-transporting P-type ATPase; EDX: Energy-Dispersive X-ray spectroscopy; LA-ICP-MS: Laser Ablation Inductively Coupled Plasma Mass Spectrometry; LM: Light Microscopic; SIMS: Secondary Ion Mass Spectrometry; SXRF: Synchrotron X-ray Fluorescence.
Introduction
Under physiological conditions, several metals and metalloids play pivotal roles. They are integral part of protein complexes, bind to nucleic acids, and act as essential cofactors or catalysts in a wide variety of reactions. Moreover, there are many examples in which metal ions are key factors that predict correct protein function, stability and structure. There are also numerous examples in which metal-containing proteins catalyze the rate-limiting step in crucial reaction chains, enhance cellular bioenergetics, inhibit cellular apoptosis, act as radical scavengers, prevent nutritional stress and suppress environmental toxicity [1,2]. There are also some metals that interact with hormones, thereby modulating their stimulatory or inhibitory activity. Moreover, in plants the development of vegetative and reproductive tissues as well as the circadian clock is significantly influenced by metals such as copper (Cu) confirming the importance of metals in a multiplicity of biological processes [3].
Based on the central position and incredible importance of trace metals, excess supply and shortcomings have serious consequences and are causative involved in disease formation in humans. Metal overload might result from hereditary disorders such as hereditary haemochromatosis ("iron overload") or Wilson's disease ("copper overload"), exorbitant ingestion, inhalation, percutaneous absorption, long-term parenteral nutrition, frequent blood transfusion, and chronic intake of metal-afflicted drugs. Beside these extrinsic causes, also organ failures (kidney, gall, pancreas, and bladder) that interfere with the secretion or clearance of a specific metal can induce unwanted internal metal bioaccumulation. Vice versa, inadequate supply during periods of extensive fasting, monotonic alimentation (Vegans), malabsorption or inadequate resorption in the gastrointestinal tract might be common causes of specific shortcomings that might manifest in specific clinical symptoms.
Paradigmatically, Wilson's disease is associated with the formation of neurological or psychiatric symptoms and hepatic copper overload. In some cases, affected persons are identified by so called Kayser-Fleischer rings that are characteristic copper deposits in the eye within the area between the cornea and the sclera. However, such clinical signs can vary from one person to another and are therefore not suitable to diagnose respective disease or to allow statements about disease progression or success of a potential therapy.
In contrast, the determination of exact metal concentrations or the quantitation of serum metal binding proteins that directly or indirectly correlate with the concentration of a specific metal are thought to be more reliable for diagnosis. In addition, the presence of metal deposits in biopsy specimens can be identified in defined histopathological stains. The most popular laboratory histopathology stain is Prussian blue that is used to identify iron deposits in tissue. However, all these methods have potential pitfalls and allow metal quantification only with moderate accuracy and reproducibility.
During the last decades, several novel and innovative biometal imaging techniques were established. Energy-dispersive X-ray spectroscopy (EDX) is a chemical microanalysis technique that relies on the detection of X-rays emitted from the sample. In EDX analysis, individual elements are identified by their specific set of peaks on its X-ray emission spectrum, thereby providing precise information on the chemical composition of subcellular structures [4]. Unfortunately, several elements have overlapping peaks in EDX that under certain circumstances prevent the assignment of a peak to a specific element. Synchrotron X-ray fluorescence (SXRF) microscopy is a non-destructive analysis technique that can collect data on multiple metals simultaneously. SXRF measures the intrinsic fluorescent properties of elements within a specimen that become released after exposure to X-rays. In this analysis, the exposure to X-rays causes the ejection of electrons from the inner shell of the atom, resulting in electron vacancies that might provoke electrons from the outer shells to relax and fill the holes. This process is associated with emission of X-ray photons that again is specific for a specific element. Moreover, the intensity of the emitted energies allows element quantitation [5]. This methodology was shown to be attractive for both experimental investigations and clinical studies [6,7]. In a third method, the secondary ion mass spectrometry (SIMS), the surface of the specimen is sprinkled with a focused primary ion beam and the ejected secondary ions are separated and identified according to their mass/charge ratios in a mass analyzer. Although the SIMS technique has a high detection sensitivity and allows analysis of small samples, samples must be vacuum compatible and quantification is often complicated. On the other side several innovative protocols were developed with the ability to generate 3D molecular images of elements in tissue samples [8] or alternatively in which elements were measured in nanometerscale (i.e. "NanoSIMS") in appropriate biological samples [9].
Laser ablation inductively coupled mass spectrometry (LA-ICPMS) has multi-element capability and allows analysing a large variety of biological materials with high spatial resolution. It can identify a large number of metals and metalloids at extremely low concentrations. The principle is based on the ablation of material from a biological sample by use of a finely focused laser beam (Figure 1). The ablated material is then transported into the inductively coupled plasma source of a mass spectrometer using an inert carrier gas stream. There it is vaporized, atomized and ionized and subsequently separated according to their mass/ charge ratio. LA-ICP-MS is appropriate to measure an element concentration at a single point within a tissue, or alternatively to perform line scans or even two- or three-dimensional imaging of individual or multiple elements or isotopes in one measurement [10,11]. At the same time, LA-ICP-MS provides good sensitivity for major, minor, trace, and ultra-trace elements with high sample throughput and without the need of wasteful sample preparation. Therefore, this technology has found nowadays a large number of biomedical applications and is on the way to be introduced in diagnostics.
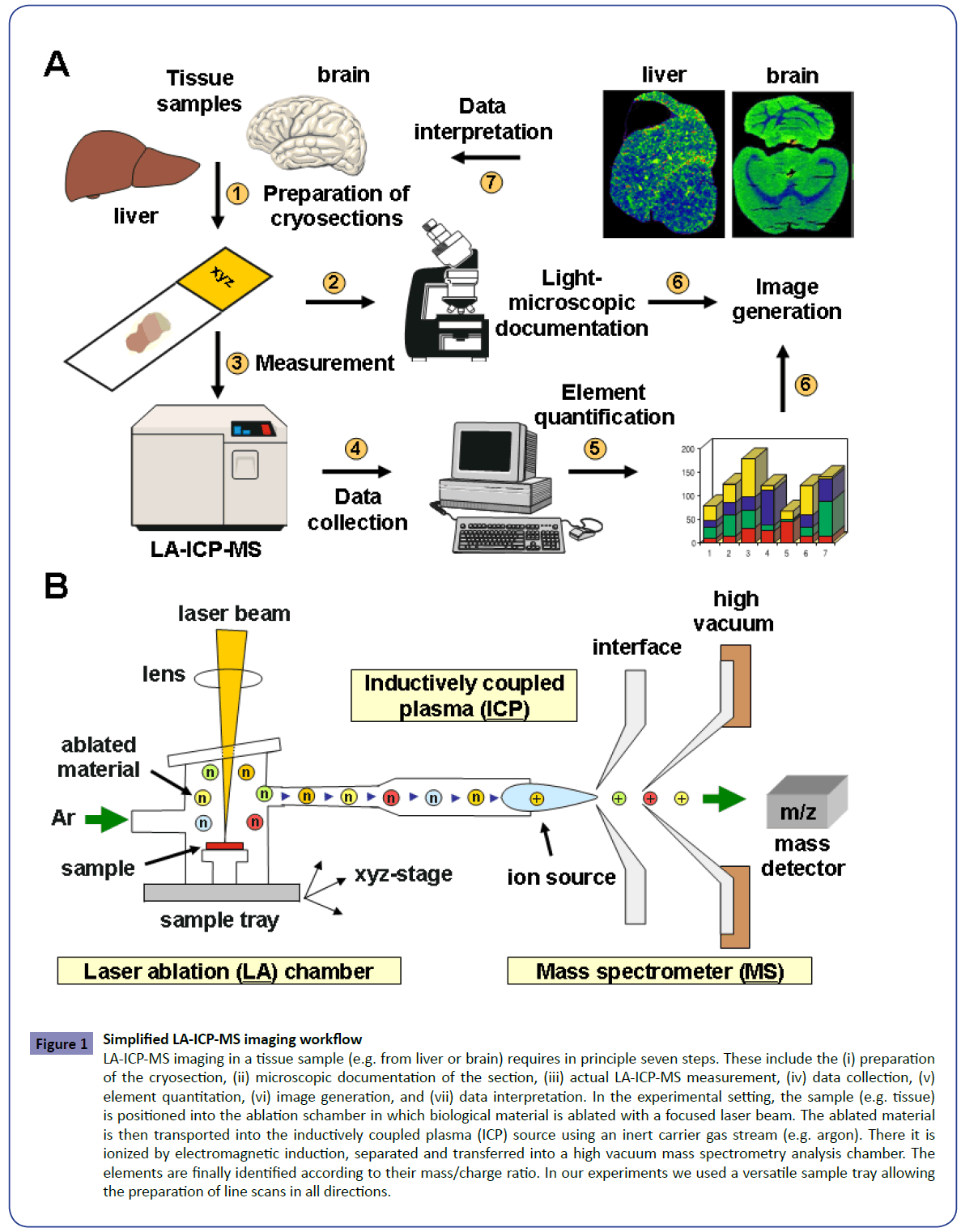
Figure 1: Simplified LA-ICP-MS imaging workflow
LA-ICP-MS imaging in a tissue sample (e.g. from liver or brain) requires in principle seven steps. These include the (i) preparation
of the cryosection, (ii) microscopic documentation of the section, (iii) actual LA-ICP-MS measurement, (iv) data collection, (v)
element quantitation, (vi) image generation, and (vii) data interpretation. In the experimental setting, the sample (e.g. tissue)
is positioned into the ablation schamber in which biological material is ablated with a focused laser beam. The ablated material
is then transported into the inductively coupled plasma (ICP) source using an inert carrier gas stream (e.g. argon). There it is
ionized by electromagnetic induction, separated and transferred into a high vacuum mass spectrometry analysis chamber. The
elements are finally identified according to their mass/charge ratio. In our experiments we used a versatile sample tray allowing
the preparation of line scans in all directions.
In the present study, we optimized and extended previous LAICP- MS studies in which imaged metals in livers and brains of wild type and Atp7b deficient mice. We demonstrate that the alterations in metal content and distribution are closely linked to the progression of the disease.
Materials and Methods
Liver and brain specimens
Liver and brain tissues were taken from wild type or Atp7b−/− mice with a genetic 129/Sv background. The gene disruption is caused by an early termination codon in the Atp7b gene introduced by a neomycin cassette-containing substitution within exon 2 of the Atp7b gene [12]. The animals used for this study were housed at a standard animal care facility, according to the guidelines of the Institutional Animal Care and Use Committees and in accordance with governmental requirements with a 12 hrs:12 hrs light to dark cycle [13]. For metal imaging in respective tissues, 30 μM thick tissue sections were prepared by cryosectioning that were mounted on adhesive glass slides.
LA-ICP-MS measurements and standardisation
The element measurements were performed in a quadrupolebased inductively coupled plasma mass spectrometer (XSeries 2, Thermo Scientific, Bremen, Germany) that was coupled to a laser ablation system (NWR 213; New Wave Research, Fremont, CA, USA). The LA-ICP-MS parameters in our measurements were set to RF power = 1400-1600 W, gas flow 0.85-1.05 L/min, isotopes measured per run = 21-29, dwell time per isotope = 0.02-0.3 sec, laser power = 50%, scan speed = 80-90 μm/sec, spot size = 30 μM, and y-distance between the centre of lines = 30 μM, respectively. All trace metal concentrations were calculated from ion intensities averaged throughout freely drawn regions of interest and depicted in μg/g tissue. The measurements were normalized to the average net 13C ion intensity that was taken as a surrogate marker of slice thickness. Because certified mouse liver and brain reference standards are not commercially available, we prepared in-house tissue standards with well-defined element concentrations by spotting the different elements to homogenized tissue extracts as previously described [14]. For more experimental details about this technology refer to previous papers of our group [14-17].
LA-ICP-MS image generation
Representative images were generated from the continuous Excel list of raw pixel values using a modified in-house LA-ICPMS Image Generation software that is based on the IMAGENA software [18]. We optimized this software by integration of Visual Basic for Applications (VBA) user-defined functions. This resulting "Excel Laser Ablation Imaging (ELAI)" software tool allows the creation of high resolution and properly scaled LA-ICP-MS images within a reasonable time[19]. Moreover, this software tool allows to transfer the generated images to other systems and export them into common image formats such as TIFF or JPG.
Results and Discussion
In our laboratory, we have recently introduced the LA-ICP-MS technology and imaged metal concentrations in brain and liver specimens obtained of experimental disease models and clinical human samples. In one study in which we compared normal and fibrotic human liver tissue we found that most of the metals are homogeneous distributed within the normal tissue, while some metals such as iron and copper are significantly redistributed within fibrotic livers [15]. We also used LA-ICP-MS to image the changes in metal distribution in a murine model of Wilson’s disease that had an engineered defect in the Atp7b gene [14]. This study revealed that the Cu accumulation within the hepatic tissue was associated with regional alterations in zinc (Zn) and iron (Fe) content. In the same animal model we also showed that Cu age-dependently accumulated throughout the brain parenchyma while it was decreased in periventricular regions [16]. In respective mice Cu overload was also demonstrated in livers and shown to be associated with simultaneous imbalances in hepatic Fe and zinc Zn [17]. Similar findings were recently confirmed in well-defined human liver samples that were obtained from patients suffering from Wilson’s disease [17].
We here optimized and extended these studies and now asked if LA-ICP-MS is suitable to identify metal imbalances that occur in hepatic tissue undergoing pathological changes, necrosis and tumorigenesis. Therefore, we imaged specimen from healthy liver tissue from wild type (control) and Atp7b deficient mice. The Atp7b gene encodes an copper-transporting P-type ATPase, which is localized in the trans-Golgi network of liver and brain. It is a key regulator that balances the copper content in the body by excreting excess copper into bile and plasma. As outlined above the genetic disruption of the Atp7b gene results in copper accumulation in tissues and lead to cerebral-induced neurological or psychiatric issues and liver failure.
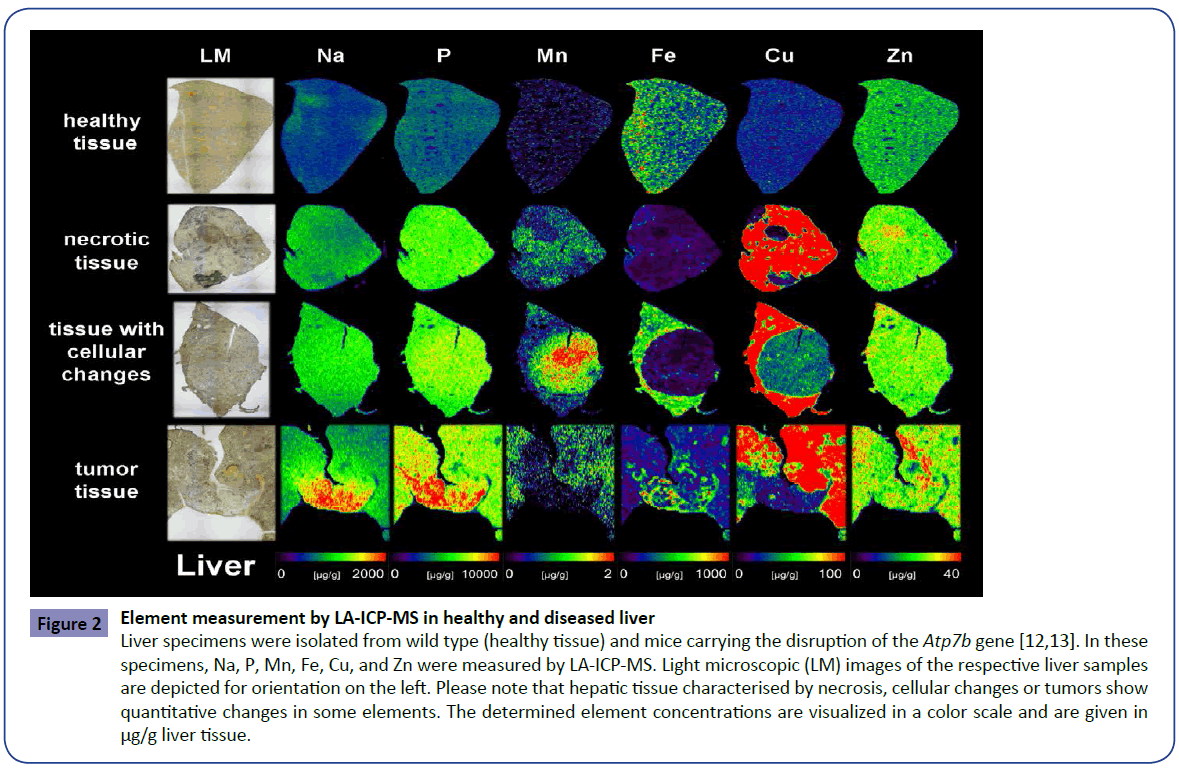
Bioimaging of metals by LA-ICP-MS in wild type animals revealed that the hepatic concentrations of sodium (Na), phosphorus (P), manganese (Mn), Cu and Zn are more or less evenly distributed within the normal tissue. The distribution of Fe that was punctual increased within the tissue was in full agreement with the architectonic structure of the liver in which Fe shows a decreasing gradient from the rim areas in the central regions of the tissue. In contrast to these findings, there is a exorbitant accumulation of Cu throughout the tissue in livers originating from animals that lack functional Atp7b protein. This Cu overload was associated with additional slight changes in intrahepatic concentrations of other metals (e.g. Zn). Interestingly, necrotic tissue areas were characterized by lowered Cu concentrations. Similar conspicuous changes affecting the concentration of Mn, Fe and Cu were also found in tissue areas enriched with pathologically altered cells (Figure 2). While the concentration of Mn was elevated in dedifferentiated tissue areas, the concentration of Fe was lowered compared to the surrounding tissue. In addition, the regional accumulation of Cu that represents the causative toxic element during Wilson's disease was reduced in dedifferentiated areas. Interestingly, respective areas were surrounded by a sharp band in which Cu showed intermediate concentrations. All these alterations had no impact of the concentrations of Na, P, and Zn.
Figure 2: Element measurement by LA-ICP-MS in healthy and diseased liver
Liver specimens were isolated from wild type (healthy tissue) and mice carrying the disruption of the Atp7b gene [12,13]. In these
specimens, Na, P, Mn, Fe, Cu, and Zn were measured by LA-ICP-MS. Light microscopic (LM) images of the respective liver samples
are depicted for orientation on the left. Please note that hepatic tissue characterised by necrosis, cellular changes or tumors show
quantitative changes in some elements. The determined element concentrations are visualized in a color scale and are given in
μg/g liver tissue.
However, in regions in which the tissue formed tumors, the concentration of all elements tested were altered in varying degrees. In particular, the concentration of Na, P and Fe were increased in the tumors, possibly reflecting the elevated metabolic activity in these areas. In contrast, the traces of Mn and Cu were decreased in the tissue surrounding the tumors. We actually do not know if these dysbalanced element concentrations within the tumors and surrounding tissue are the cause for tumor formation or a consequence of the altered metabolism present within the altered tissue. However, this data indicate that the identification of altered metal concentrations might be potentially interesting to diagnose malignant tissue transformation or to score hepatic disease stages.
In another set of experiments, we extended a former study on cerebral metal measurements in Atp7b deficient mice [16]. In particular, we tried to enhance the resolution of our images to raise the significance of our previous results. To do so, we sectioned a brain of a two years old Atp7b deficient mice and a respective age-matched control. In respective sections, we then comparatively determined the content of Na, P, Mn, Fe, Cu and Zn (Figure 3). While we observed only negligible differences in the content of Na and P, there was an excessive accumulation of Cu throughout the brain parenchyma, while the concentration of Cu were not increased proportionally in the periventricular regions. Moreover, the cerebral Cu overload was associated with alterations in cerebral content of other metals (e.g. Mn and Zn), while the concentration of other metals (Fe) showed only marginal changes. As we have already discussed before [16], this high cerebral Cu accumulation in special brain areas and resulting cognitive alterations during Wilson's disease might be the consequence of differential regional susceptibility to Cu within the brain. Of course, our data further support the notion that the Atp7b deficient mice are a good experimental model to study the cerebral Cu accumulation and that LA-ICP-MS is a powerful and innovative analytical technique suitable to display the complex changes in metal composition of the tissue in a highly vivid manner.
Figure 3: LA-ICP-MS imaging in healthy and diseased brain sections
Brain sections were prepared from normal (upper panels) and Atp7b deficient mice (lower panels) at ages of approximately 24 months.
Na, P, Mn, Fe, Cu, and Zn were imaged and quantified by LA-ICP-MS. The LM images on the left are depicted for orientation. Please
note that the accumulation of Cu in the brain of the Atp7b-/- is not uniform and preferentially affects specialized brain areas and that
the increase in Cu is also associated with alterations in concentration and distribution of other elements. The determined element
concentrations are visualized in a color scale and are given in μg/g brain tissue. More information about the sample preparation and
analysis of time-dependent alterations in cerebral metal content in Atp7b null mice is provided elsewhere [16].
In addtion, LA-ICP-MS offers an endless variety of other options in biomedical research and diagnostics. Our experience in the last years has convinced us that the results obtained in LA-ICP-MS are accurate and mostly independent of potential pre-analytical deficiencies that may arise from sample collection or processing. Measurements can be performed with high sample throughput. It further allows real-time measuring and quantification of metals, metalloids and selected non-metals simultaneously. Based on all these favourable features, it can be expected that in future LA-ICP-MS measurements are not only extremely attractive for experimental studies, but also will be a good diagnostic option for clinical practice in cases where a biopsy (e.g. from the liver) is already taken during clinical care of a patient. At the end, we hope that these commented LA-ICP-MS examples will inspire scientists and clinicians to apply this extraordinary technique in research and diagnostics.
Funding
RW is supported by grants from the German Research Foundation (SFB TRR57, P13) and the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the RWTH Aachen University (IZKF E7-6).
Acknowledgements
The authors thank Uta Merle (Department of Gastroenterology, University Hospital Heidelberg, Heidelberg, Germany) for making available mouse probes and Astrid Zimmermann (Central Institute of Engineering, Electronic und Analytics (ZEA-3), Research Centre Jülich (FZJ), Jülich, Germany) for excellent technical assistance.
Competing Interests
There is no competing interests and the authors have nothing to declare.
References
- Sharma S, Rais A, Sandhu R, Nel W, Ebadi M (2013) Clinical significance of metallothioneins in cell therapy and nanomedicine. Int J Nanomedicine 8: 1477-1488.
- Susnea I, Weiskirchen R (2015) Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom Rev.
- Peñarrubia L, Romero P, Carrió-Seguí A, Andrés-Bordería A, Moreno J, et al. (2015) Temporal aspects of copper homeostasis and its crosstalk with hormones. Front Plant Sci 6: 255.
- Wyroba E, Suski S, Miller K, Bartosiewicz R (2015) Biomedical and agricultural applications of energy dispersive X-ray spectroscopy in electron microscopy. Cell MolBiolLett 20:488-509.
- Singh UM, Sareen P, Sengar RS, Kumar A (2013) Plant ionomics: a newer approach to study mineral transport and its regulation. ActaPhysiol Plant 35:2641-2653.
- Punshon T, Ricachenevsky FK, Hindt MN, Socha AL, Zuber H (2013) Methodological approaches for using synchrotron X-ray fluorescence (SXRF) imaging as a tool in ionomics: examples from Arabidopsis thaliana. Metallomics 5:1133-1145.
- Braidy N, Poljak A, Marjo C, Rutlidge H, Rich A, et al. (2014) Metal and complementary molecular bioimaging in Alzheimer's disease. Front Aging Neurosci 6:138.
- Fletcher JS (2015) Latest applications of 3D ToF-SIMS bio-imaging. Biointerphases 10:018902.
- Gao D, Huang X, Tao Y (2015) A critical review of NanoSIMS in analysis of microbial metabolic activities at single-cell level. Crit Rev Biotechnol.
- Becker JS, Matusch A, Becker JS, Wu B, Palm C, et al. (2011) Mass spectrometric imaging (MSI) of metals using advanced BrainMet techniques for biomedical research. Int J Mass Spectrom 307:3-15.
- Becker JS (2013) Imaging of metals in biological tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): state of the art and future developments. J Mass Spectrom 48:255-268.
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, et al. (1999) Null mutation of the murine Atp7b (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8:1665-1671.
- Sauer SW, Merle U, Opp S, Haas D, Hoffmann GF, et al. (2011) Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b-/- mice as a model for Wilson disease. BiochimBiophysActa 1812:1607-1615.
- M M P, Merle U, Weiskirchen R, Becker JS (2013) Bioimaging of copper deposition in Wilson’s diseases mouse liver by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MSI). Int J Mass Spectrom 354-355C:281-287.
- M M P, Weiskirchen R, Gassler N, Bosserhoff AK, Becker JS (2013) Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS One 8:e58702.
- Boaru SG, Merle U, Uerlings R, Zimmermann A, Weiskirchen S, et al. (2014) Simultaneous monitoring of cerebral metal accumulation in an experimental model of Wilson's disease by laser ablation inductively coupled plasma mass spectrometry. BMC Neurosci 15:98.
- Boaru SG, Merle U, Uerlings R, Zimmermann A, Flechtenmacher C, et al. (2015) Laser ablation inductively coupled plasma mass spectrometry imaging of metals in experimental and clinical Wilson's disease. J Cell Mol Med 19:806-814.
- Osterholt T, Salber D, Matusch A, Becker JS, Palm C (2011) IMAGENA: Image Generation and Analysis - an interactive software tool handling LA-ICP-MS data. Int J Mass Spectrom 30.
- Uerlings R, Matusch A, Weiskirchen R (2016) Reconstruction of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) spatial distribution images in Microsoft Excel 2007. Int J Mass Spectrom 395: 27-35..
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences