Post-Treatment Circulating Free HPV DNA As a Marker of Treatment Outcome in Patients with HPV-Related Propharyngeal Cancer After Radio(chemo)therapy
Rutkowski TW,Mazurek AM, Śnietura M, Wygoda A, Pigłowski W3, Kołosza Z, Składowski K1and Widłak P
DOI10.21767/2573-5365.100035
Rutkowski TW1*, Mazurek AM2, ÃÆââ¬Â¦Ãâà ¡nietura M3, Wygoda A1, PigÃÆââ¬Â¦Ãâââ¬Å¡owski W3, KoÃÆââ¬Â¦Ãâââ¬Å¡osza Z4, SkÃÆââ¬Â¦Ãâââ¬Å¡adowski K1 and WidÃÆââ¬Â¦Ãâââ¬Å¡ak P2
1 Department of Radiooncology, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, Wybrzeze Armii Krajowej 15, 44- 101 Gliwice, Poland
2 Center for Translational Research and Molecular Biology of Cancer, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, Wybrzeze Armii Krajowej 15, 44- 101 Gliwice, Poland.
3 Tumor Pathology Department, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, Wybrzeze Armii Krajowej 15, 44- 101 Gliwice, Poland
4 Department of Epidemiology, Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, Wybrzeze Armii Krajowej 15, 44- 101 Gliwice, Poland
- *Corresponding Author:
- Tomasz Wojciech Rutkowski, MD, PhD
Department of Radiooncology
Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology
Gliwice Branch, Wybrzeze Armii Krajowej 15, 44-101, Gliwice, Poland.
Tel: 48-32-231-9628
E-mail: tomr22@tlen.pl
Received Date: June 28, 2017; Accepted Date: July 26, 2017; Published Date: July 28, 2017
Citation: Rutkowski TW, Mazurek AM, ÃÆââ¬Â¦Ãâà ¡nietura M, Wygoda A, PigÃÆââ¬Â¦Ãâââ¬Å¡owski W, et al. (2017) Post-Treatment Circulating Free HPV DNA As a Marker of Treatment Outcome in Patients with HPV-Related Propharyngeal Cancer After Radio(chemo)therapy. Cell Mol Med. Vol. 3 No. 2:12
Copyright: © 2016 Kumar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Circulating cell-free HPV16 DNA (cfHPV DNA) assessed after treatment completion was tested as a prognostic marker of treatment failure in patients with HPVrelated oropharyngeal cancer (OPC) treated with radiotherapy alone (RT) or radiotherapy combined with chemotherapy (CHRT). Fifty-five patients with OPC in whom cfHPV DNA was found prior to RT/CHRT were included into the study. cfHPV DNA was subsequently assessed after treatment completion. cfHPV DNA remission (cfHPVrem) was defined as the absence of cfHPV DNA after treatment completion. The association between cfHPVrem and the risk of local, nodal or distant failure was calculated. Survival curves were calculated according to the Kaplan-Meier technique and compared according to cfHPVrem after treatment completion with the log-rank test. Thirteen (24%) patients presented no cfHPVrem after treatment completion. For these patients, the risk of nodal failure was significantly higher (HR=9.38, 95% CI: 1.63-53.97, p=0.009) than for patients with cfHPVrem. The probability of dissemination was comparable in patients with cfHPVrem and with no cfHPVrem (p=0.95). For patients with HPV related OPC, who has no cfHPVrem after RT/CHRT completion stricter follow up is proposed due to higher risk of nodal failure.
Keywords
Circulating-free HPV DNA; Head and neck cancer; Radichemotherapy; Liquid biopsy
Introduction
Locoregional relapse is the main reason of treatment failure in patients with head and neck squamous cell carcinoma (HNSCC) who underwent radiotherapy (RT) alone or combined with chemotherapy (CHT). Despite treatment intensification for locoregionally advanced HNSCC, approximately half of the treated population returns with recurrent or refractory disease and for these patients, the 1-year survival rate ranges from 5% to 33% with a median OS of 6 months to 9 months [1,2]. Early detection of treatment failure is essential for successful salvage [3] but challenging due to the lack of reliable markers and low sensitivity of imaging methods directly after treatment [4,5]. A causal association between human papillomavirus (HPV) and a subset of HNSCC especially oropharyngeal cancer (OPC) has changed a previous dogma as to epidemiology, pathology and clinical course of HNSCC. Recent studies showed that circulating free HPV DNA (cfHPV DNA) could be found in blood of most patients with HPV-related OPC and that the amount of cfHPV DNA changes according to treatment progress and may be related to treatment response [6-8]. If the response to treatment is reflected in cfHPV DNA level at the end of treatment, such ‘liquid biopsy’ [9] may become a feasible marker for early prediction of treatment results. Despite an increasing rate of HPV-related OPC, that has been recently observed [10], research data considering cfHPV DNA as a marker of treatment results is still poor. In this paper, the role of the cfHPV16 DNA (cfHPV DNA) assessment after treatment completion as a marker of treatment outcome in patients with HPV16-related OPC has been presented.
Materials and Methods
Newly diagnosed patients with OPC who had been admitted to the Radiation Oncology Department at Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology in Gliwice between September 2012 and May 2016 were eligible for inclusion to the study. Patients with known metastatic disease or immune suppression were not eligible. At the time of study recruitment (presentation to the institution), informed consent was obtained from all the patients. The project was approved by the Bioethics Committee at the Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology. The study conforms with the Code of Ethics of the World Medical Association. Plasma of 179 patients was obtained for cfHPV DNA assessment prior to any treatment. Circulating free HPV DNA was found in 55 (31%) patients and subsequently serial cfHPV DNA blood samples were taken after induction CHT (indCHT) for those who underwent indCHT and directly after the treatment completion in all the patients. HPV-related tumor etiology was confirmed in tissue samples in 47 (84%) cases from this group. For 8 (16%) patients tissue samples were not available or not informative. The group consisted of 28 (52%) men and 27 (48%) women at the mean age of 55 years (range: 30 years to 75 years). In 5 (9%) patients metastatic neck lymph nodes had been dissected as a diagnostic procedure prior to the presentation at the Radiation Oncology Department. All the patients were treated definitively with RT or RT combined with CHT in 6 (11%) and 49 (89%) cases respectively. Induction chemotherapy was performed in 30 (53%) patients. It was followed with RT alone in 8 (14%) or with radiochemotherapy (CHRT) in 22 (39%) cases. In 2 (5%) patients after indCHT palliative RT was given due to progressive disease. Clinical data in details is presented in Table 1.
| Case number | Sex | HPV in tissue | TN | indCHT (no of courses) |
Radiotherapy (Gy) |
cfHPVrem after: indCHT/treatment completion | Treatment results |
Follow-up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | Positive | T3N3 | 4 | CHRT(70) | 0/0 | NED | 46 |
| 2 | M | Positive | T4N1 | 0 | CHRT(70) | -/1 | NED | 42 |
| 3 | M | Positive | T2N2 | 3 | CHRT(70) | 0/0 | NED | 47 |
| 4 | F | Positive | T3N3 | 3 | RT(20) | 1/1 | LRF | 6 |
| 5 | M | Positive | T3N3 | 3 | CHRT(70) | 0/0 | NED | 46 |
| 6 | F | Positive | T4N1 | 0 | CHRT(70) | -/1 | NED | 9 |
| 7 | F | N/A | T3N1 | 0 | CHRT(70) | -/0 | NED | 44 |
| 8 | M | Positive | T3N2 | 0 | RT (72) | -/0 | NED | 27 |
| 9 | F | Positive | T4N2 | 1 | RT(60) | 0/0 | NED | 41 |
| 10 | M | Positive | T4N1 | 0 | RT (60) | -/0 | NED | 37 |
| 11 | M | Positive | T3N3 | 4 | CHRT(70) | 0/0 | NED | 33 |
| 12 | M | Positive | T1N3 | 1 | CHRT(66) | 0/0 | NED | 36 |
| 13 | M | N/A | T2N3 | 4 | RT (72) | 0/0 | DM | 6 |
| 14 | F | Positive | T3N2 | 3 | RT(70) | 0/0 | NED | 30 |
| 15 | F | Positive | T4N2 | 3 | RT (20) | 1/1 | LRF | 23 |
| 16 | F | Positive | T4N2 | 3 | CHRT(70) | 0/0 | NED | 34 |
| 17 | F | Positive | T4N2 | 3 | CHRT(70) | 0/0 | RF | 34 |
| 18 | F | Positive | T4N2 | 2 | CHRT(70) | 0/0 | NED | 22 |
| 19 | M | Positive | T2N3 | 3 | CHRT(70) | 1/0 | NED | 31 |
| 20 | M | Positive | T2N2 | 0 | CHRT(70) | -/0 | NED | 35 |
| 21 | F | N/A | T2N2 | 0 | RT (66) | -/0 | NED | 35 |
| 22 | F | N/A | T2N2 | 0 | CHRT(70) | -/0 | NED | 16 |
| 23 | M | Positive | T4N2 | 0 | CHRT(70) | -/0 | NED | 5 |
| 24 | M | Positive | T3N2 | 4 | CHRT(70) | 1/0 | DM | 24 |
| 25 | M | N/A | T3N2 | 3 | CHRT(70) | 1/0 | NED | 28 |
| 26 | F | Positive | T4 | 1 | CHRT(70) | 0/0 | LF | 18 |
| 27 | M | Positive | T4N3 | 1 | RT(70) | 1/1 | RF,DM | 23 |
| 28 | M | Positive | T3N2 | 2 | CHRT(70) | 0/0 | NED | 23 |
| 29 | F | Positive | T3N3 | 3 | CHRT(70) | 1/1 | RF | 19 |
| 30 | F | Positive | T1pN2 | 0 | CHRT(70) | -/0 | NED | 22 |
| 31 | M | N/A | T3N1 | 3 | CHRT(70) | 1/0 | NED | 21 |
| 32 | F | Positive | T1pN3 | 0 | CHRT(70) | -/1 | NED | 22 |
| 33 | F | N/A | T2pN2 | 0 | RT(66) | -/0 | DM | 17 |
| 34 | F | Positive | T1N2 | 0 | RT(70) | -/0 | NED | 22 |
| 35 | F | Positive | T1N2 | 2 | CHRT(70) | 1/1 | NED | 13 |
| 36 | F | Positive | T4N0 | 0 | CHRT(70) | 0/1 | NED | 13 |
| 37 | M | N/A | T2N3 | 3 | CHRT(70) | 0/0 | NED | 11 |
| 38 | M | Positive | T3N2 | 3 | CHRT(70) | 1/1 | NED | 12 |
| 39 | M | Positive | T3N2 | 0 | CHRT(70) | -/1 | NED | 13 |
| 40 | M | Positive | T2N2 | 0 | CHRT(70) | -/0 | NED | 12 |
| 41 | M | Positive | T2N2 | 0 | CHRT(70) | -/0 | NED | 14 |
| 42 | F | N/A | T1N2 | 1 | CHRT(70) | 0/0 | NED | 11 |
| 43 | F | Positive | T3N2 | 2 | CHRT(70) | 0/0 | NED | 9 |
| 44 | F | Positive | T4N1 | 0 | CHRT(70) | -/1 | NED | 9 |
| 45 | M | Positive | T1pN2 | 0 | CHRT(70) | -/0 | NED | 6 |
| 46 | M | Positive | T2N0 | 0 | RT(70) | -/0 | NED | 11 |
| 47 | M | Positive | T2N2 | 3 | CHRT(70) | 0/0 | NED | 7 |
| 48 | M | Positive | T2N2 | 0 | RT(70) | -/1 | NED | 10 |
| 49 | F | Positive | T2N2 | 3 | RT(70) | 1/0 | NED | 4 |
| 50 | F | Positive | T3N3 | 3 | RT(70) | 0/0 | NED | 5 |
| 51 | F | Positive | T1N2 | 0 | CHRT(70) | -/0 | NED | 6 |
| 52 | M | Positive | T1pN2 | 0 | CHRT(70) | -/0 | NED | 4 |
| 53 | M | N/A | T1N2 | 1 | CHRT(70) | 1/0 | NED | 4 |
| 54 | F | Positive | T1N2 | 0 | CHRT(70) | -/0 | NED | 2 |
| 55 | F | Positive | T2N2 | 0 | CHRT(70) | -/0 | NED | 3 |
N/A: data not available; indCHT: Induction Chemotherapy; RT: Radiotherapy; CHRT: Radiochemotherapy; 0: cfHPVrem; 1: detectable cfHPV DNA; NED: No evidence of disease; RF: Regional Failure; LF: Local Failure, LRF: Locoregional Failure, DM: Distant Metastases
Table 1: Patients characteristic according to clinical factors, treatment and treatment results according to cfHPV DNA status after indCHT and after treatment completion.
Plasma samples collection and DNA extraction
Peripheral blood (12 ml) was collected into K3EDTA tubes (Becton-Dickinson, New Jersey, Franklin Lakes, USA). Plasma was immediately (up to 1 hour) separated by double centrifugation at 300 × g and 1000 × g, both at 4°C for 10 minutes. Aliquots of the plasma were frozen at -80°C. DNA was extracted (according to the manufacturer’s instructions) from 1 ml of plasma by the Genomic Mini AX Body Fluids kit (A&A Biotechnology, Gdynia, Poland) and dissolved in 20 μl of the elution buffer.
Analysis of the total cfDNA and HPV16 in blood plasma
For the total cfDNA (circulating cell-free DNA) measurement amplification of TERT (human telomerase reverse transcriptase) was used as described previously [11]. Shortly, each measurement consisted of a standard curve, negative control and a sample. For the construction of the standard curve, we used a control genomic DNA. The concentration of the total cfDNA was between 0.118 ng/μl and 1.130 ng/μl. The concentration of the total cfDNA did not influence the cfHPV DNA detection as we presented before [11-16]. For HPV16 detection primers and probe sequence were designed for E6/7 region. PCR was done in a final volume of 30 μl using the TaqMan Genotyping Master Mix (Applied Biosystems, CA, Foster City, USA). If HPV16 was found, the confirmation of its presence was done on the second independent DNA isolation. Complete remission of cfHPV DNA (cfHPVrem) was defined as not detectable cfHPV DNA in plasma after the treatment.
Tumour samples: Detection of HPV and confirmation of its biological activity in the tumour tissue
DNA was isolated from formalin-fixed paraffin-embedded (FFPE) tumour samples using the mSample Preparation System DNA (Abbott Laboratories, Abbott Park, Illinois, USA) (N=33). Detection of HPV in FFEP tumour samples was made using a Realtime high-risk HPV test (Abbott Molecular, Abbott Park, Illinois, USA). The reactions contained 50 ng of extracted DNA. Samples were considered to be positive when the Ct value, which means the number of cycles of which the fluorescence exceeded the threshold, was less or equal to the cut-off value of 35. Samples for which the β-globin Ct value was >35, were considered to be non-informative.
Statistical Analysis
Due to the treatment method patients were divided into these who underwent indCHT and those who did not. Locoregional recurrence free survival was defined as a time with no evidence of the disease in primary tumor site (local recurrence free survival) and regional lymph nodes (nodal recurrence free survival). Metastases free survival was defined as a time with no evidence of distant spread of the disease. The rate of treatment failure according to cfHPVrem after the treatment completion was calculated and compared with Fisher’s exact test. Survival curves were calculated according to the Kaplan-Meier technique and compared according to cfHPVrem after the treatment completion with the log-rank test.
Results
Treatment outcome according to cfHPV DNA status after treatment completion
Among 30 (53%) patients who underwent indCHT, six patients had 1 course, four patients had 2 courses, sixteen patients had 3 courses and four patients had 4 courses of indCHT (Table 1). Less than 3 courses of indCHT were given due to poor toleration in five cases, or total or almost total disease remission after one or two courses in the remaining five patients. The number of courses of indCHT did not influence the rate of cfHPVrem neither after indCHT, nor after the treatment completion. cfHPVrem after the treatment completion was found in 8 (80%) and 16 (80%) patients who received 1 or 2 and 3 or 4 courses of indCHT respectively (p=1.0). Twelve patients out of 30 (36.5%) still had virus in the blood (no cfHPVrem) after indCHT, (patients 4, 15, 19, 24, 25, 27, 29, 31, 35, 38, 49, 53). Six patients from this group had subsequently no cfHPVrem after the treatment completion (patients 4, 15, 27, 29, 35, 38). Treatment failure was finally observed in 5 patients (patients 4, 15, 24, 27, 29) what is 42% of those who had no cfHPVrem after indCHT. Ultimately treatment failure was found in four patients (4, 15, 27, 29) out of six (67%) who had no cfHPVrem after the treatment completion. The risk of treatment failure in patients after indCHT who had no cfHPVrem after the induction was higher than in the others and significantly higher if no cfHPVrem was still observed after the treatment completion (p=0.029).
Among 25 patients who underwent RT or CHRT without indCHT in 18 (72%) cfHPVrem was found after the treatment completion and 7 patients had no cfHPVrem at that moment (patients 2, 6, 33, 36, 39, 44, 48). Only 1 patient from this group finally failed (patient 33).
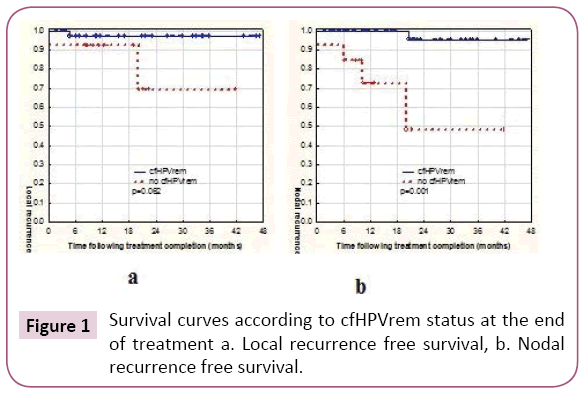
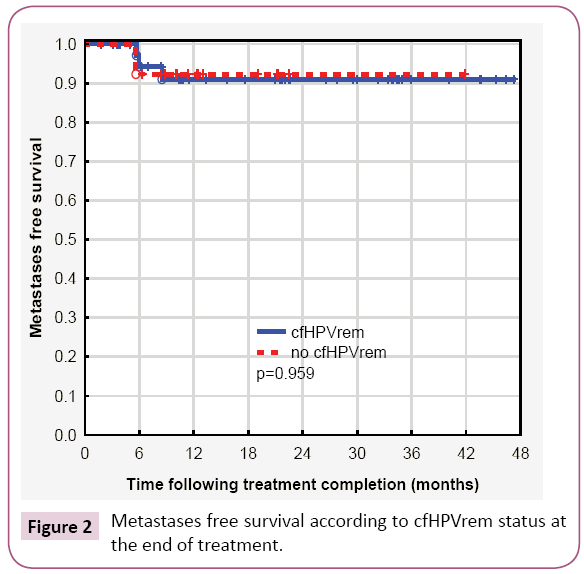
Considering together patients with indCHT and without it, in 13 (24%) patients no cfHPVrem was found after the treatment completion. The risk of subsequent failure was lower in patients with cfHPVrem after the treatment completion (12%) compared to the patients with no cfHPVrem (31%). The distribution of failures (local, nodal and distant) is presented in Table 2. Local failure was found in three patients. Local recurrence free survival was higher in patients with no cfHPVrem at the end of the treatment (p=0.062) (Figure 1a). Patient 4 was not cured remaining with bulky local and regional disease. Local recurrence of disease was also found in patient 26 after 5 months of complete disease remission and in patient 15, simultaneously with nodal recurrence, after 22 months of complete disease remission. Nodal failure was found in patients 4 and 15 (simultaneously with local failure mentioned above) and in patients 17, 27 (who simultaneously developed metastases to the lung) and 29, after 21 months, 6 and 10 months of complete disease remission respectively. In one case, metastatic squamous cell carcinoma after salvage lymphadenectomy has been confirmed, in other two cases metastatic cancer cells were found in the fine needle aspiration. One patient denied histopathological verification of lymph nodes with FDG uptake and also the further treatment. Patients with no cfHPVrem at the end of the treatment had over twenty times higher risk of nodal failure (HR=21.88, 95% CI: 2.33-205.19, p=0.0069) (Figure 1b). In patients 13, 24, 27 (with simultaneous nodal failure) and 33 PET scanning revealed metastatic disease in lung (patient 24, 27, 33) and liver (patient 13) 6 months and 8 months after treatment completion respectively. Patients with no cfHPVrem at the end of the treatment had similar probability of dissemination as these with cfHPVrem (p=0.95) (Figure 2). Second primary (SP) was found in 3 (5%) patients. It appeared in patient 23 after 7 months (gastric cancer), in patient 9 after 12 months (lung cancer), and in patient 2 after 20 months (pancreatic cancer) after the treatment. cfHPVrem was found in these patients.
| Variables | Local failure* | Nodal failure* | Distant failure* | Cured |
|---|---|---|---|---|
| no cfHPVrem | 2 (66.7%) | 4 (80.0%) | 1 (25.0%) | 9 (19.6%) |
| cfHPVrem | 1 (33.3%) | 1 (20.0%) | 3 (75.0%) | 37 (80.4%) |
| p | 0.122 | 0.01 | 1.0 | -- |
*separately calculated patients with more than one site of failure (patients 4, 15, 27)
Table 2: The distribution of failure (local, nodal and distant) according to cfHPV status.
Discussion
Results of our study indicate that for the patients with HPV related OPC in whom cfHPV DNA is present prior to RT/CHRT, post-treatment cfHPV DNA status has a prognostic value. Viral DNA has been disappearing quickly in the blood of most patients during treatment but not in all cases. cfHPV DNA was still detectable in almost one fourth of all patients after the treatment completion. In this group with the exception of one patient who was not cured and one with residual neck mass who was finally cured all the others presented no evidence of disease at that moment. However, despite of total clinical clearance of disease, higher risk of the treatment failure could be expected in patients with no cfHPVrem at the end of the treatment. Thirtyone per cent of patients with virus detectable at the end of treatment (no cfHPVrem) ultimately failed, while among these with cfHPVrem-only in 12% failed. Among those who underwent indCHT (more intense therapy), the treatment failure was observed even in 67% of patients without cfHPVrem after the treatment completion. Our results indicate that persisting HPV DNA after the treatment completion may help to predict rather locoregional failure, especially nodal, but not distant spread of disease. Patients without cfHPVrem after treatment presented over 20 times higher risk of nodal failure than the patients with cfHPVrem while not difference was found in the risk of metastatic disease between both groups. Ahn et al. presented similarly adverse results of persistent HPV DNA in serum of patients after the treatment. Patients with posttreatment plasma HPV-positive status demonstrated a significant association with increased risk of disease recurrence after adjusting for other risk factors like alcohol use, smoking status, and N classification. In their study, among 5 patients with detectable serum HPV DNA after treatment 4 (75%) developed subsequently recurrence while only 2 from 30 patients (13%) who did not have HPV detected in posttreatment plasma sample [17]. Cao et al. followed small group of 14 patients who presented pretreatment detectable plasma HPV DNA in serial plasma samples (weekly or every other week) during the course concurrent chemoradiotherapy. There was a gradual decline in HPV DNA during the therapy which, disappeared at all at the end of it. There were no obvious differences in the rate of HPV DNA decline between the patients with eventual tumour relapse and those without it. Four of patients however eventually relapsed: one locoregionally and three distantly in the lungs [6]. It shows, similarly as in our study, that HPV DNA status at the end of the treatment may not be a good predictor of distant spread of the disease. Most data on the role of viral cfDNA as a prognostic marker comes from studies reporting DNA of Epstein- Barr virus (EBV) in patients who underwent treatment due to nasopharyngeal carcinoma (NPC). Circulating EBV DNA has been tested as a marker for NPC since 1998 [18,19] and correlates positively with disease stage and exhibits prognostic value in patients with NPC [20]. The drop in viral DNA in the blood during RT/CHRT was accompanied by remission of NPC as a response to treatment [21]. Patients with more rapid clearance of EBV DNA from the circulation responded better to treatment and had a better survival probability [22,23]. Due to high sensitivity and specificity [24,25], plasma EBV DNA has been adopted as a marker for clinical management of patients with NPC [26]. It was suggested that high circulating plasma EBV DNA load assessed 6 weeks post-primary treatment may predict a subsequent relapse [27-29]. For patients treated due to NPC persisting, increased, post-treatment viral DNA load or a subsequent rise after a previous drop seems to be an indication for PET/CT scanning for high risk of relapse [30]. Campitelli et al. showed an analysis of sequential serum specimens in patients with cervical cancer. The concentration of cfHPV preceded radiological relapse in one case and was associated with an 8-mm liver metastasis in the other one. The authors hypothesized that tumour DNA might be more easily released from tumour tissue corresponding to a relapse or metastasis than to a primary lesion. They concluded that during follow-up, cfHPV DNA concentration could be a highly specific surrogate marker for minimal residual disease or subclinical relapse [31]. In another study, in patients treated due to cervical cancer, plasma cfHPV DNA became undetectable after the treatment in those who had complete response and showed no evidence of disease after the treatment. Contrary to that, cfHPV DNA was still present after the treatment and during the followup in those who had persistent disease after the treatment or presented recurrence. Authors concluded that plasma cfHPV DNA might be a valuable noninvasive marker for monitoring the therapeutic response and disease progression in patients treated due to cervical cancer [32]. Some limitations may restrict conclusion that could be drawn from this study. Only patients with HPV DNA in the blood prior to the treatment were followed with the subsequent assessment of HPV DNA. It is likely that more HPV-positive patients release HPV DNA to the blood but had not been caught at the moment of our assessment prior to the treatment. In further research, all the patients with HPV-related tumorus (based on a tissue sample) should be followed with HPV DNA assessment to recognize “HPV DNA plasma windows”. Our group consisted of patients with OPC exclusively and the usefulness of HPV DNA assessment in HPV-related tumours of other sites in head and neck region has not been explored yet.
Conclusion
In summary, for patients with HPV-related OPC, cfHPV DNA seems to be a marker for active cancer. If cfHPV DNA is still present in the blood after the treatment completion it may indicate patients with higher risk of nodal failure. For such patients, stricter followup should be considered. Further research is needed however to confirm our results in a larger group of patients.
Funding
AM Mazurek: grant NN402450039 funded by National Science Centre in Poland.
References
- PignonJP, Le MaîtreA, MaillardE (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. RadiotherOncol92: 4-14.
- AngKK, HarrisJ, WheelerR (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med: 24-35
- Goodwin WJ Jr (2000) Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: When do the ends justify the means? Laryngoscope 110: 1-18.
- TantiwongkosiB, Yu F, KanardA (2014) Role of (18)F-FDG PET/CT in pre-andpost-treatment evaluation in head and neck carcinoma. World J Radiol 6: 177-191.
- AndradeRS, HeronDE, DegirmenciB (2006) Posttreatment assessment of response using FDG-PET/CT for patients treated with definitive radiation therapy for head and neck cancers. Int J RadiatOncolBiolPhys65: 1315-1322.
- Cao H, BanhA, KwokS (2012) Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int JRadiatOncolBiol Phys 82: 351-358.
- CaponeRB, Pai SI, Koch WM (2000) Detection and quantitation of human papillomavirus (HPV) DNA in the sera of patients with HPV-associated head and neck squamous cell carcinoma. Clin Cancer Res 6: 4171-4175.
- Dahlstrom KR, Li G, Hussey CS (2015) Circulating human papillomavirus DNA as a marker for disease extent and recurrence among patients with oropharyngeal cancer. Cancer 121:3455-3464.
- Heitzer E, Ulz P, Geigl JB(2015) Circulating tumor DNA as a liquid biopsy for cancer. ClinChem 61: 112-123.
- Goodman MT, Saraiya M, Thompson TD (2015) Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer 51: 2759-2767.
- MazurekAM, Rutkowski T, Fiszer-Kierzkowska A (2016) Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol 54: 36-41.
- Dias FL (2013) Assessment of treatment response after chemoradiation of head and neck cancer. CurrOncol Rep 15: 119-127.
- De Bree R, Van Der Putten L, Brouwer J (2009) Detection of locoregional recurrent head and neck cancer after (chemo)radiotherapy using modern imaging. Oral Oncol 45: 386-393.
- McCollum AD, Burrell SC, Haddad RI (2004) Positron emission tomography with 18Ffluorodeoxyglucose to predict pathologic response after induction chemotherapy and definitive chemoradiotherapy in head and neck cancer. Head Neck 26: 890-896.
- ImaizumiA, Yoshino N, Yamada I(2006) A potential pitfall of MR imaging for assessing mandibular invasion of squamous cell carcinoma in the oral cavity. Am J Neuroradiol27: 114-122.
- Guo T, Qualliotine JR, Ha PK (2015) Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 121: 1977-1984.
- Ahn SM, Chan JY, Zhang Z (2014) Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol Head Neck Surg 140: 846-854.
- Rettig EM, Wentz A, Posner MR (2015) Prognostic Implication of persistent human papillomavirus Type 16 DNA detection in oral rinses for human papillomavirus-related oropharyngeal carcinoma. JAMA Oncol 1: 907-915.
- Mutirangura A, Pornthanakasem W, Theamboonlers A(1998) Epstein-Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin Cancer Res 4: 665-669.
- Lo YM, Chan LY, Lo KW (1999) Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 59: 1188-1191.
- Yip TT, Ngan RK, Fong AH (2014) Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol 50: 527-538.
- Hsu CL, Chang KP, Lin CY (2012) Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck 34: 1064-1070.
- Wang WY, TwuCW, Chen HH (2010) Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res 16: 1016-1024.
- Leung SF, Tam JS, Chan AT (2004) Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein-Barr virus DNA and anti-Epstein-Barr viral capsid antigen IgA antibody. ClinChem 50: 339-345.
- Leung SF, Zee B, Ma BB (2006) Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J ClinOncol 24: 5414-5418.
- Chan KC, Lo YM(2007) Circulating tumour-derived nucleic acids in cancer patients: Potential applications as tumour markers. Br J Cancer 96: 681-685.
- Chan AT, Ma BB, Lo YM (2004) Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: Therapeutic monitoring with plasma Epstein-Barr virus DNA. J ClinOncol 22: 3053-3060.
- Lo YM, Chan LY, Chan AT (1999) Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res 59: 5452-5545.
- Chang KP, Hsu CL, Chang YL (2008) Complementary serum test of antibodies to Epstein-Barr virus nuclear antigen-1 and early antigen: A possible alternative for primary screening of nasopharyngeal carcinoma. Oral Oncol 44: 784-792.
- Wang WY, Twu CW, Lin WY (2011) Plasma Epstein-Barr virus DNA screening followed by ¹ÃÆâÃâÃÂÃâøF-fluoro-2-deoxy-D-glucose positron emission tomography in detecting posttreatment failures of nasopharyngeal carcinoma. Cancer 117: 4452-4459.
- Campitelli M, Jeannot E, Peter M (2012) Human papillomavirus mutational insertion: Specific marker of circulating tumor DNA in cervical cancer patients. PLoS One 7: 433-493.
- Yang HJ, Liu VW, Tsang PC (2004) Quantification of human papillomavirus DNA in the plasma of patients with cervical cancer. Int J Gynecol Cancer 14: 903-910.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences