Editorial Note On Cellular & molecular medicine: Open Access
Ao Li
DOI10.21767/2573-5365.100038
Ao Li*
Department of Pharmacology, Yale University School of Medicine, New Haven, CT 06520, USA
- *Corresponding Author:
- Ao Li
Department of Pharmacology, Yale
University School of Medicine
New Haven, CT 06520, USA
Tel: 203-507-0301
E-mail: ao.li@yale.edu
Received Date: November 20, 2017 Accepted Date: December 07, 2017 Published Date: December 11, 2017
Citation: Li A (2017) Present Status and Advances in Autosomal Dominant Polycystic Kidney Disease. Cell Mol Med. Vol.3 No.3:15 doi: 10.21767/2573-5365.100038
Keywords
ADPKD; Cystogenesis; PKD1; PKD2; Cell signaling
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic disorder which is characterized by progressive fluid-filled cysts in the kidney. It occurs worldwide and in all races with significant different prevalence in different region: United States (1/400) [1], Denmark (1/1000) [2], Walsh (1/2459) [3], Japan (1/4033) [4]. Most recent statistics indicated that the prevalence of ADPKD in Europe is estimated to be 1/2500 [5]. ADPKD is the fourth most common single cause of end-stage renal failure (ESRD), which occurs in 50% of affected individuals by the age of 60 years [6]. The high risk factors that affects the progression ESRD include PKD1 mutation [7], bigger Total Kidney Volume (TKV) [8], younger diagnosed age, early onset of hypertension [9], proteinuria and microalbuminuria [10,11].
ADPKD is a systemic disease with various extrarenal manifestations including hypertension [12,13], liver and pancreatic cysts [14], intracranial aneurysms [15], aortic root and thoracic aorta abnormalities [16-18], mitral valve prolapse [19,20] and abdominal wall hernias [21,22]. 50% to 70% of patients with ADPKD display hypertension, mostly occurring before they develop a significant decrease in renal function [23,24]. Up to 83% of ADPKD patients have hepatic cysts. However, there is no overt clinical symptoms and rarely cause liver failure [25,26]. The prevalence of intracranial aneurysms (ICA) among ADPKD patients is about 12% [27]. A family history of intracranial hemorrhage can significantly increase the risk of ICA [28]. Therefore, examination and evaluation of extrarenal organ of ADPKD patients is also very important.
At present, there is no safe and effective treatment of ADPKD in clinic. Therefore, understanding the pathogenesis of ADPKD may provide a new therapeutic target for the clinical treatment and lay the foundation for the development of various biological and drug treatments for ADPKD.
PKD Genes and Proteins
ADPKD is genetically heterogeneous with two genes identified, PKD1 [ch16p13.3, 46 exons] and PKD2 [ch4q21, 15 exons], which encode the proteins polycystin-1 [PC1] and polycystin-2 [PC2], respectively. Amongst ADPKD patients, 85-90% of cases result from mutations in PKD1, while another 10-15% of cases are caused by mutations in PKD2 [29]. Polycystin-1 is a transmembrane mechano/chemo-sensor receptor and polycystin-2 is a nonselective cationic channel belongs to the subfamily of transient receptor potential (TRP) channels. Polycystin-1 contains 4302 amino acids and consists of a huge extracellular domain, 11 transmembrane domains and a shorter cytoplasmic tail domain [30,31]. Polycystin-1 is found in most segments of the nephron and is distributed in a variety of subcellular structures such as the primary cilia, cytoplasmic vesicles, plasma membrane at focal adhesions, desmosomes, adherens junctions, and possibly endoplasmic reticulum and nuclei. Among them, polycystin-1 distributed in primary cilia is considered to play an important role in the mechano/chemo-sensor function [32-34], whereas polycystin-1 distributed in the side wall and the basal body participates in the formation of intercellular adhesion and focal adhesion [35]. In addition, a cleavage product of polycystin-1 that includes the C-terminal tail can translocate to the nucleus and regulate gene transcription [36,37].
Polycystin-2 (968 amino acids) contains a short N-terminal cytoplasmic region with a ciliary targeting motif, 6 transmembrane domains, and a short C-terminal portion [38,39]. Polycystin-1 physically interacts with polycystin-2 through a coiled-coil domain in the C-terminal portion which forms the polycystin-signaling complex that play a role in chemosensory or mechanosensory signal transduction [40]. Disruption of both gene products results in similar clinical phenotypes. Polycystin-2 is distributed in all nephrons except glomeruli which subcellular localized in the endoplasmic reticulum and cilia [41]. Polycystin-2 is a nonselective cation channel belonging to the transient receptor potential cation channel family [42]. It regulates intracellular Ca2+ levels and affects various cellular activities such as cell proliferation and differentiation and epithelial cell polarity [43-45].
ADPKD and Cilia
Primary cilia are microtubule-based antenna-like organelles protruding from the surface of vertebrate cells that mediate a number of signaling pathways during development and tissue homeostasis [32]. Defects in primary cilia are thought to play an important role in polycystic kidney disease [46,47]. According to the current hypothesis, primary cilium is a kind of sensory organelle. Polycystin-1 and polycystin-2 co-localized in primary cilia forming PC complex through the C-terminal cytoplasmic tail domain [40]. PC1 exert the role of mechano/chemo-sensor, response to mechanical or fluid-induced cilia bending, and modulates Ca2+ channel activity of polycystin-2, causing changes in intracellular Ca2+ levels [48]. Growth and maintenance of primary cilia relies on the Intraflagellar Transport (IFT) system [49,50], which transports various proteins to and from cilia, including polycystin-1 and polycystin-2, via kinesin and dynein [51-53]. Thus, besides the absence of polycystins that leads to polycystic kidney disease phenotype, IFT system deletions such as deactivating the IFT system components Kif3a, Ift20 and Ift88 in mouse models also cause polycystic kidney disease phenotype [54]. Although cilia loss is associated with cystic kidney disease, recent studies have shown that there is no evidence of primary cilia-associated Ca2+ influx in both the renal tubules and cell lines stimulated by fluid flow at physiological levels [55]. Therefore, the role and function of fibril in ADPKD remains to be further studied and elucidated.
Discussion
Signaling pathways as targets in PKD treatment
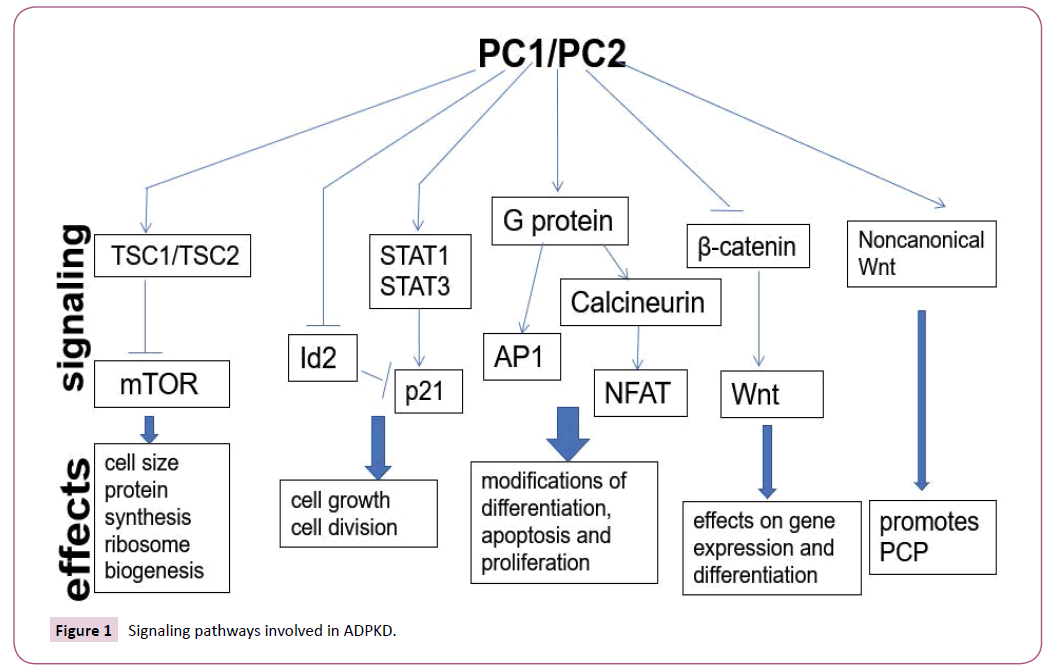
Many signaling pathways control ADPKD cyst formation such as the mammalian target of rapamycin (mTOR) [56,57], cyclic adenosine monophosphate (cAMP) [58,59], Wnt signaling pathway [44,60], G protein coupled receptor [GPCR] [61] and Signal Transducer and Activator of Transcription (STAT) signaling pathway [62] (Figure 1).
mTOR pathway
The serine/threonine kinase mammalian target of rapamycin (mTOR) is an evolutionary conserved signaling molecule, integrating a broad spectrum of signals and environmental cues, including oxidative stress, availability of nutrients and energy. Under physiological conditions, growth and proliferation requires both an appropriate stimulus and a favorable environment [63]. In contrast, malignant (cancer) and benign human proliferative disorders including ADPKD are frequently accompanied by aberrant activation of the mTOR signaling pathway [57,64,65]. In ADPKD, deletion of polycystin-1 in tubular epithelial cells results in decreased expression of the TSC1/TSC2 complex, which in turn reduces mTOR inhibition, resulting in over activation of mTOR and subsequent abnormal proliferation of epithelial cells [66]. Results from animal experiments also show that mTOR inhibitors are able to slow the increase of kidney volume in ADPKD models of ADPKD [67].
cAMP pathway
As a second messenger, cyclic adenosine monophosphate (cAMP) involved in cell proliferation, differentiation, DNA synthesis and is capable of regulating a number of cellular biological processes, including electrolyte and humoral transport. The level of cAMP is determined by the activity of adenylyl cyclase (AC) and phosphodiesterase (PDE), both of which mediate the synthesis and degradation of cAMP and are regulated by Ca2+. Elevated intracellular cAMP levels were observed in PKD patients and in various animal models of PKD [58,68]. Increased levels of cAMP in ADPKD renal epithelial cells activates the B-Raf/MEK/ ERK signaling pathway [69,70] and promotes cell proliferation. Accordingly, treatment of vasopressin V2 receptor antagonist (Tolvaptan) which reduce cAMP level in ADPKD animal models and patients exhibits slowed renal volume increase and improved renal function [71,72]. However, but the resulting damage to liver function and other toxicities (such as polyuria) severely limit the wide range of clinical applications of this drug.
Wnt pathway
Wnt signaling pathway play pivotal role in cell proliferation, migration, apoptosis and organ development, including the kidneys. There are two main types of Wnt signaling pathways: canonical Wnt signaling and non-canonical Wnt signaling. Recent studies have demonstrated that human cystic disease may involve Wnt signal transduction [60,73]. Transgenic mouse for β-catenin exhibits severe PKD phenotypes, showing that the upregulation of β-catenin alone is sufficient to induce cyst formation in the kidney [74]. Other reports demonstrated that PC1 CTT colocalizes with and binds to b-catenin in the nucleus. The PC1 CTT inhibits the ability of both b-catenin and Wnt ligands to activate TCF-dependent gene transcription, which is a major effector of the canonical Wnt signaling pathway [75]. Our work also found that loss of PC2 can increase the expression of b-catenin, axin2 and c-Myc. This suggests that PC2 may play a function role in inhibition of β-catenin-dependent Wnt signaling [44]. All of these results indicated that have shown that the loss of Pkd1 and Pkd2 account for abnormal activation of Wnt signaling pathways and cystogenesis.
G protein signaling pathway
Although there is a difference between the structure of PC1 protein and the classical G protein-coupled receptor (GPCR), the GPS site near the extracellular domain of polycystin-1 play similar biological function of GPCR. Disruption this site result in the formation of renal cysts in mice [76]. Polycystin-1 also directly binds to the G protein subunit and activates downstream molecules such as c-Jun N-terminal kinase, AP-1 transcription factor, and activated T cell signaling cascade nuclear factor which regulate normal cell proliferation differentiation and apoptosis [77,78]. In ADPKD, polycystin complex inhibits PC1-mediated G protein signaling pathway activation. Loss of polycystins give rise to activation of G protein signaling pathway thereby promotes cyst formation [79]. In addition, G-protein-signaling modulator 1 (GPSM1) is able to upregulate the channel function of the polycystin complex via the G protein subunit. In the Pkd1 mouse model, complete deletion of GPSM1 promotes cyst formation and reduces renal function [80], indicating that GPSM1 may also play a role in GPCR activation of ADPKD.
Signal Transducer and Activator of Transcription (STATs)
JAK/STAT signal pathway is a cytokine-stimulated signal transduction pathway involved in many important biological processes such as cell proliferation, differentiation, apoptosis and immune regulation. Studies have shown that polycystin-1 participate in the regulation of JAK/STAT signaling pathway. PC1 activates STAT3 more under the mediation of JAK2 and thus regulates DNA transcriptional activity of epithelial cells [37]. STAT3 is significantly activated in cyst-lining epithelial cells of human ADPKD patients and mouse model as well as in the developing and postnatal kidneys, but inactivated in mature kidneys. These results demonstrated that the STAT3 signaling pathway is regulated by PC1 and serve as a driver of renal epithelial proliferation during normal kidney development and renal cyst growth. Studies have shown that inhibition of STAT6 by leflunomide (LEF) slows cyst growth in ADPKD mouse models [81].
Conclusion and Future Perspectives
Currently, abnormal proliferation, loss of polarity of epithelial cells and abnormal secretion of fluid are the three key components of ADPKD cyst formation [82]. The activation of mTOR, GPCR and Wnt pathways promote the abnormal proliferation of epithelial cells. The activation of cAMP/CFTR pathway increases the fluid secretion in the cyst. In-depth study of these mechanisms is expected to help people find potential targets for the treatment of ADPKD. At present, there are some clinical trials targeting different molecules of these pathways. However, either poor efficacy or intolerant side effects limit widespread use in clinic. Therefore, the discovery of new therapeutic targets and new drugs based on various molecules and signaling pathways will have great practical significance in the clinical treatment of ADPKD.
References
- Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, et al. (1983) Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota during 1935-1980. Am J Kidney Dis 2: 630-639.
- Dalgaard OZ (1957) Bilateral polycystic disease of the kidneys a follow-up of two hundred and eighty-four patients and their families. Acta medica Scandinavica Supplementum 328: 1-255.
- Davies F, Coles GA, Harper PS, Williams AJ, Evans C, et al. (1991) Polycystic kidney disease re-evaluated: A population-based study. Q J Med 79: 477-485.
- Horie S, Mochizuki T, Muto S, Hanaoka K, Fukushima Y, et al. (2016) Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol 20: 493-509.
- Willey CJ, Blais JD, Hall AK, Krasa HB, Makin AJ, et al. (2016) Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrology, dialysis, transplantation: Official publication of the European Dialysis and Transplant Association - European Renal Association.
- Paul BM, Vanden Heuvel GB (2014) Kidney: Polycystic kidney disease. Wiley Interdiscip Rev Dev Biol 3: 465-487.
- Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, et al. (2006) Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. JASN 17: 3013-3019.
- Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, et al. (2006) Volume progression in polycystic kidney disease. N Engl J Med 354: 2122-2130.
- Chapman AB, Stepniakowski K, Rahbari-Oskoui F (2010) Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 153-163.
- Chapman AB, Johnson AM, Gabow PA, Schrier RW (1994) Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. JASN 5: 1349-1354.
- Johnson AM, Gabow PA (1997) Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol 8: 1560-1567.
- Perrone RD, Malek AM, Watnick T (2015) Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 11: 589-598.
- Marlais M, Cuthell O, Langan D, Dudley J, Sinha MD, et al. (2016) Hypertension in autosomal dominant polycystic kidney disease: a meta-analysis. Arch Dis Child Educ Pract Ed 101: 1142-1147.
- Yazdanpanah K, Manouchehri N, Hosseinzadeh E, Emami MH, Karami M, et al. (2013) Recurrent acute pancreatitis and cholangitis in a patient with autosomal dominant polycystic kidney disease. Int J Prev Med 4: 233-236.
- Ring T, Spiegelhalter D (2007) Risk of intracranial aneurysm bleeding in autosomal-dominant polycystic kidney disease. Kidney International 72: 1400-1402.
- Ong AC (2009). Screening for intracranial aneurysms in ADPKD. BMJ 339: 3763.
- Rozenfeld MN, Ansari SA, Mohan P, Shaibani A, Russell EJ, et al. (2016) Autosomal dominant polycystic kidney disease and intracranial aneurysms: Is there an increased risk of treatment. AJNR Am J Neuroradiol 37: 290-293.
- Adeola T, Adeleye O, Potts JL, Faulkner M, Oso A (2001) Thoracic aortic dissection in a patient with autosomal dominant polycystic kidney disease. J Natl Med Assoc 93: 282-287.
- Lumiaho A, Ikaheimo R, Miettinen R, Niemitukia L, Laitinen et al. (2001) Mitral valve prolapse and mitral regurgitation are common in patients with polycystic kidney disease type 1. Am J Kidney Dis 38: 1208-1216.
- Oflaz H, Alisir S, Buyukaydin B, Kocaman O, Turgut F, et al. (2005) Biventricular diastolic dysfunction in patients with autosomal-dominant polycystic kidney disease. Kidney International 68: 2244-2249.
- Li L, Szeto CC, Kwan BC, Chow KM, Leung CB, et al. (2011) Peritoneal dialysis as the first-line renal replacement therapy in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 57: 903-907.
- Lucon M, Ianhez LE, Lucon AM, Chambo JL, Sabbaga E, et al. (2006) Bilateral nephrectomy of huge polycystic kidneys associated with a rectus abdominis diastasis and umbilical hernia. Clinics 61: 529-534.
- Chapman AB, Johnson A, Gabow PA, Schrier RW (1990) The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med 323: 1091-1096.
- Ecder T, Schrier RW (2001) Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol 12: 194-200.
- Torres VE (2007) Treatment of polycystic liver disease: One size does not fit all. Am J Kidney Dis 49: 725-728.
- Bae KT, Grantham JJ (2010) Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96-106.
- Xu HW, Yu SQ, Mei CL, Li MH (2011) Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke 42: 204-206.
- Pirson Y, Chauveau D, Torres V (2002) Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 13: 269-276.
- Peters DJ, Breuning MH (2001) Autosomal dominant polycystic kidney disease: Modification of disease progression. Lancet 358: 1439-1444.
- Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, et al. (1995) The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151-160.
- Nims N, Vassmer D, Maser RL (2003) Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: Evidence for 11 membrane-spanning domains. Biochemistry 42: 13035-13048.
- Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK (2009). The primary cilium as a complex signaling center. Current biology 19: 526-535.
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129-137.
- Scheffers MS, Van der Bent P, Prins F, Spruit L, Breuning MH, et al. (2000) Polycystin-1, the product of the polycystic kidney disease 1 gene, co- localizes with desmosomes in MDCK cells. Hum Mol Genet 9: 2743-2750.
- Wilson P (2004) Polycystic kidney disease. N Engl J Med 350.
- Chauvet V, Tian X, Husson H, Grimm DH, Wang T, et al. (2004) Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433-1443.
- Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, et al. (2006) Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Developmental cell 10: 57-69.
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, et al. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339-1342.
- Celic A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ (2008) Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem 283: 28305-12.
- Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, et al. (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179-183.
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, et al. (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119: 1383-1395.
- Gonzalez-Perret S, Kim K, Ibarra C, Damiano AE, Zotta E, et al. (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proceedings of the National Academy of Sciences of the USA. Proc Natl Acad Sci USA 98: 1182-1187.
- Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, et al. (2005) Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol 7: 1102-1112.
- Kim I, Ding T, Fu Y, Li C, Cui L, et al. (2009) Conditional mutation of Pkd2 causes cystogenesis and upregulates beta-catenin. J Am Soc Nephrol 20: 2556-2569.
- Chun JN, Lim JM, Kang Y, Kim EH, Shin YC et al. (2014) A network perspective on unraveling the role of TRP channels in biology and disease. Pflugers Arch 466: 173-182.
- Waters AM, Beales PL (2011) Ciliopathies: An expanding disease spectrum. Pediatr Nephrol 26: 1039-1056.
- Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis and disease. Cell 137: 32-45.
- Gallagher AR, Germino GG, Somlo S (2010) Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118-130.
- Jonassen JA, San Agustin J, Follit JA, Pazour GJ (2008) Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183: 377-384.
- Silverman MA, Leroux MR (2009) Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol 19: 306-316.
- Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508-2516.
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12.
- Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, et al. (2007) Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol 179: 501-514.
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, Van Groen T, et al. (2007) Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr biol 17: 1586-1594.
- Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, et al. (2016) Primary cilia are not calcium-responsive mechanosensors. Hum Mol Genet 531: 656-660.
- Pema M, Drusian L, Chiaravalli M, Castelli M, Yao Q, et al. (2016) mTORC1-mediated inhibition of polycystin-1 expression drives renal cyst formation in tuberous sclerosis complex. Nat Commun 7.
- Li A, Fan S, Xu Y, Meng J, Shen X, et al. (2017) Rapamycin treatment dose-dependently improves the cystic kidney in a new ADPKD mouse model via the mTORC1 and cell-cycle-associated CDK1/cyclin axis. J Cell Mol Med 21: 1619-1635.
- Hanaoka K, Guggino WB (2000) cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol 11: 1179-1187.
- Torres VE, Harris PC (2014) Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25: 18-32.
- Simons M, Mlodzik M (2008) Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42: 517-540.
- Kurbegovic A, Kim H, Xu H, Yu S, Cruanes J (2014) Novel Functional Complexity of Polycystin-1 by GPS Cleavage In Vivo: Role in Polycystic Kidney Disease. Mol Cell Biol 34: 3341-3353.
- Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, et al. (2011) Polycystin-1 regulates STAT activity by a dual mechanism. Proceedings of the National Academy of Sciences of the USA 108: 7985-7990.
- Cuyas E, Corominas-Faja B, Joven J, Menendez JA (2014) Cell Cycle Regulation by the Nutrient-Sensing Mammalian Target of Rapamycin (mTOR) Pathway. Methods Mol Biol 1170: 113-144.
- Fumarola C, Bonelli MA, Petronini PG, Alfieri RR (2014) Targeting PI3K/AKT/mTOR pathway in non-small cell lung cancer. Biochem Pharmacol 90: 197-207.
- Watnick T, Germino GG (2010) mTOR inhibitors in polycystic kidney disease. N Engl J Med 363: 879-881.
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, et al. (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proceedings of the National Academy of Sciences of the USA103: 5466-5471.
- Shillingford JM, Piontek KB, Germino GG, Weimbs T (2010) Rapamycin Ameliorates PKD Resulting from Conditional Inactivation of Pkd1. J Am Soc Nephrol 21: 489-497.
- Starremans PG, Li X, Finnerty PE, Guo L, Takakura A, et al. (2008) Mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5' end of Pkd1. Kidney Int.
- Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, et al. (2003) Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983-1994.
- Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, et al. (2007) Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157-165.
- Gattone VH 2nd, Wang X, Harris PC, Torres VE (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9: 1323-1326.
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, et al. (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407-2418.
- Benzing T, Simons M, Walz G (2007) Wnt signaling in polycystic kidney disease. JASN. 18: 1389-1398.
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, et al. (2001) Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972-5981.
- Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, et al. (2008) Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17: 3105-3017.
- Qian F, Boletta A, Bhunia AK, Xu H, Liu L, et al. (2002) Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proceedings of the National Academy of Sciences of the USA 99: 16981-16986.
- Arnould T, Kim E, Tsiokas L, Jochimsen F, Gruning W, et al. (1998) The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem 273: 6013-6018.
- Chapin HC, Caplan MJ (2010) The cell biology of polycystic kidney disease. J Cell Biol 191: 701-710.
- Delmas P, Nomura H, Li X, Lakkis M, Luo Y, et al. (2002) Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem 277: 11276-11283.
- Kwon M, Pavlov TS, Nozu K, Rasmussen SA, Ilatovskaya DV, et al. (2012) G-protein signaling modulator 1 deficiency accelerates cystic disease in an orthologous mouse model of autosomal dominant polycystic kidney disease. Proceedings of the National Academy of Sciences of the USA109: 21462-21467.
- Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, et al. (2011) Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proceedings of the National Academy of Sciences of the USA108: 18067-18072.
- Mochizuki T, Tsuchiya K, Nitta K (2013) Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol 17: 317-326.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences