The Multistep Functions of EMMPRIN/ CD147 in the Tumor Angiogenesis
Zhenzhen Wang, Zhenghao Zhao, Ting Jiang, Yanke Chen and Chen Huang
DOI10.21767/2573-5365.100012
Zhenzhen Wang1, Zhenghao Zhao1, Ting Jiang1, Yanke Chen1* and Chen Huang1,2*
1Department of Genetics and Cell Biology, PR China
2Key Laboratory of Environmentally and Genetically Associated Diseases, Xian Jiaotong University Health Science Center, Xian, PR China
- *Corresponding Author:
- Yanke Chen
Department of Genetics and Cell Biology, Xian Jiaotong University Health Science Center, Yanta Western Road 76, Xian, Shaanxi, 710061, PR China
Tel: +86-29-82657497
E-mail: paincyk@mail.xjtu.edu.cn
- Chen Huang
Department of Genetics and Cell Biology, Xian Jiaotong University Health Science Center, Yanta Western Road 76, Xian, Shaanxi, 710061, PR China
Tel: +86-29-82657497
E-mail: hchen@mail.xjtu.edu.cn
Received date: February 04, 2016; Accepted date: March 19, 2015; Published date: March 23, 2016
Citation: Wang Z, Zhao Z, Jiang T. The Multistep Functions of EMMPRIN/CD147 in the Tumor Angiogenesis. Cell Mol Med. 2016, 2:1.
Abstract
CD147 is a member of the immunoglobulin family of receptors. Both clinical studies and basic research have indicated that elevated CD147 expression is correlated with tumor progression. It facilitates cancer invasion and angiogenesis. Targeting CD147 in cancer appears a promising future therapeutic strategy, but requires a better understanding of its mode of action and regulation. This review focuses on the most recent findings that addressing the role of CD147 in tumor angiogenesis and highlight the relational mechanisms. Supporting these findings are evidences that CD147 is an important modulator in tumor angiogenesis, thus, more attractive as a target for anti-tumor treatment.
Keywords
Tumor; Angiogenesis; Leukocytes
Introduction
Tumor is one of the common diseases that seriously threaten human health. High invasion and metastasis are the principle reasons for the high mortality of cancer. Numerous studies have demonstrated that tumor vascularization is crucial in tumor progression [1-4]. EMMPRIN/CD147, a cell adhesion molecule highly expressed in a variety of tumors, is associated with poor prognosis in cancer patients. The results of our study and several other laboratories have confirmed that CD147 induces tumor angiogenesis and promotes tumor growth and metastasis. In the following pages, this review will summarize the mechanisms of CD147 induce tumor angiogenesis [5,6]. The present studies have shown that CD147 plays an important role in many aspects of the process of tumor angiogenesis.
Formation of Tumor Vessels: Vasculogenesis and Angiogenesis
The current concept was believed that, in tumors, new blood vessel formation has been thought to occur primarily via angiogenesis and vasculogenesis [2,7-15]. Angiogenesis is defined as the sprouting and growth of new capillaries from nearby existing blood vessels, however, postnatal vasculogenesisis defined as the endothelial precursor cells (EPCs) move to an angiogenic site and integrate into newly forming vessels [16-20]. In the angiogenic process, blood vessels grow through sprouting and intussusception, in which interstitial cellular columns are inserted into the lumen of pre-existing vessels and partition the vessel lumen [21]. In classical sprouting angiogenesis, the endothelial cells to form capillary walls secrete proteases which degrade the surrounding basement membrane, subsequently, invade the adjacent tissues and proliferate to generate new blood vessels.
Role of EMMPRIN/CD147 in Tumor Angiogenesis
Extracellular matrix metalloproteinase inducer (EMMPRIN), also known as CD147 and Basigin (Bsg) is a glycosylated transmembrane protein that belongs to the immunoglobulin (Ig) superfamily [22-25]. It is broadly expressed at varying levels on many cell types, including epithelial and endothelial cells, leukocytes [26-29], stromal fibroblasts [30], differentiated macrophage, activated T cells [31-34], dendritic cell [35], cardiac myocytes and platelets [36-38]. Interestingly, CD147 is highly enriched on the surface of malignant tumor cells. Indeed, CD147 has been greatly implicated in malignancy as it is highly expressed in most tumor tissues and its expression often correlates with tumor progression [39-44]. Therefore, much attention has been focused on this molecule that plays a key role in tumor angiogenesis.
Enriched CD147 in tumor cells initiates the formation of an angiogenesis niche
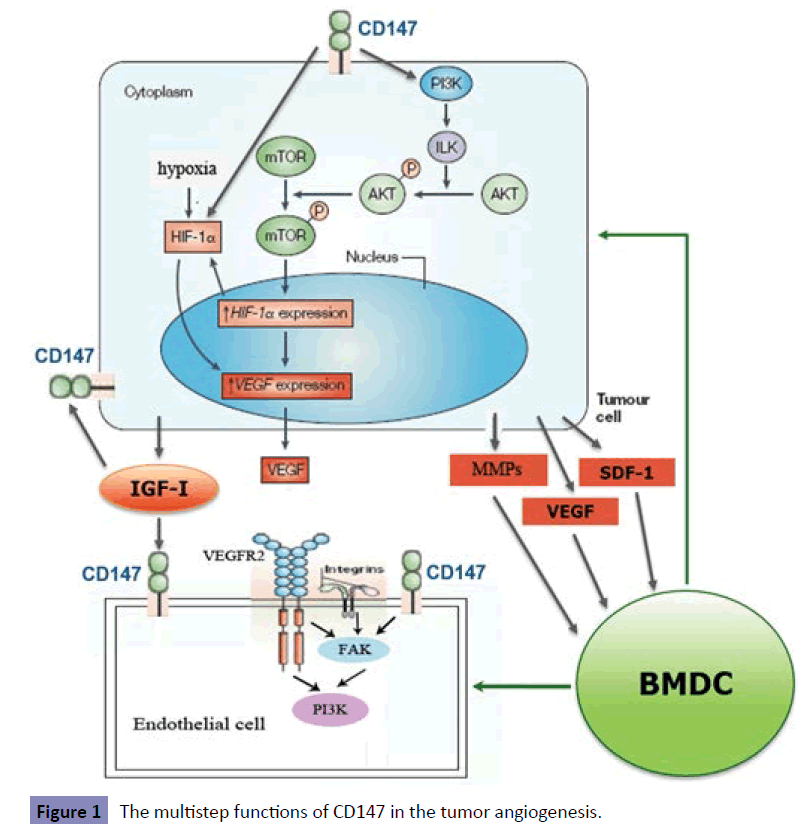
Angiogenesis is the result of the combination of signaling molecules and tumor microenvironment [45-48]. The angiogenic switch is defined as an imbalance between pro- and antiangiogenic molecules and is a key step in tumor angiogenesis [49- 53]. The angiogenic molecules include: matrix metalloproteinases (MMPs), basic fibroblast growth factors (bFGF), vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), insulin-like growth factor (IGF), epidermal growth factor (EGF), placental growth factor (PLGF) and so on [54-56]. All these molecules promote the formation of new blood vessels in tumor and stromal cells. Recently, various studies have demonstrated that CD147 regulates the cross-talk between tumors and endothelial cells (ECs), and may promote tumor angiogenesis via regulating the tumor-stromal microenvironment which contains pro-angiogenic molecules such as MMPs, HIF-1, IGF-I, VEGF and CD147 itself [57-62]. Among them, HIF-1, a key hypoxia response transcription factor, induces the expression of many target genes including VEGF and IGF. VEGF is expressed by alternative splicing as six different isoforms (VEGF121, VEGF145, VEGF165, VEGF183, VEGF189 and VEGF206). Faten Bougatef, et al. have observed that CD147 selectively increases the expression of VEGF121 and VEGF165 isoforms, but not the VEGF189 [60]. In addition, IGF-I strongly induces the expression of CD147 in HUVECs cells and multiple tumor cell lines, which shows that there is a positive feedback mechanism between CD147 and IGF-I.
Up-regulation of CD147 in HUVECs enhances the angiogenesis
Except for tumor cells, CD147 is also expressed at different levels in many other cell types, such as ECs. Mutin and colleagues demonstrated that CD147 is a surface molecular marker on HUVECs with a moderate expression level under resting condition [62]. Danilo Millimaggi showed that CD147 stimulates pro-angiogenic activities of human umbilical vein endothelial cells (HUVECs) in a CD147-dependent fashion [61]. Y. Chen reported that CD147 is significantly up-regulated in activated HUVECs and the up-regulation of this molecule strongly enhances the angiogenic phenotype of activated HUVECs. CD147 regulates angiogenesis of ECs by several mechanisms including proliferation, survival, migration, MMPs secretion and PI3K/Akt activation [29]. In addition, another study by Faten Bougatef reported that CD147 up-regulates the expression of VEGFR-2, which is a receptor of VEGF in endothelial cells. The increase in VEGFR-2 is responsible for CD147 stimulating of the migratory and tube formation capacity of ECs, thus directly regulating the angiogenic process.
CD147 recruits BMDCs and enhances tumor vasculogenesis andangiogenesis
The role of bone marrow-derived cells (BMDCs) in angiogenesis and vasculogenesis has been documented by multiple studies [16,62-66]. However, the contribution of bone marrow– derived circulating endothelial progenitor cells (CEPs) to tumor angiogenesis has been controversial, because of their low numbers in blood vessels of untreated tumors. These BMDCs appear to promote angiogenesis by the release of pro-angiogenic molecules at sites of neovascularization to stimulate expansion of local blood vessels [17,20]. In 2001, David Lyden showed that transplantation of wild-type bone marrow (BM) or VEGF-mobilized stem cells restore tumor angiogenesis and growth. This study demonstrated that recruitment of VEGF-responsive BM-derived precursors is necessary and sufficient for tumor angiogenes [16]. Brock A Peters demonstrated that bone marrow stem cells definitely contribute to tumor angiogenesis in diverse human tumor types, but this contribution is relatively small [63]. Recently, by studying GFP expressing BMDC donor cells in LLC tumor xenografts, Chen et al. noticed that CD147 regulates tumor growth and metastasis by recruitment of BMDCs, which contributes to tumor neovascularization [67]. This report revealed a novel mechanism that CD147 promotes tumor growth and metastasis by recruitment of BMDCs through controlling secretion and paracrine signaling of SDF-1 and VEGF. Until now several studies reveal a novel mechanism by which CD147 promotes tumor angiogenes via recruitment of BMDCs.
Conclusion
After reviewing the literature on this field is clear that CD147 plays a key role in tumor angiogenesis. It is highly expressed on the surface of tumor cells and facilitates to form a microenvironment induced angiogenesis. CD147 stimulates tumor cells, nearby fibroblasts and endothelial cells to produce and secrete MMP, VEGF, IGF-1, and SDF-1. They modulate the cross-talk between tumor cells and endothelial cells and BDMCs. On one hand, these growth factors activate endothelial cells and promote it proliferation, migration and neovascularization; on the other hand, these molecular signaling and recruitment of BMDCs contribute to tumor vasculogenesis and angiogenesis. In addition, IGF-1 strongly induces the expression of CD147 in HUVECs cells and tumor cells. It can feedback up-regulate production of proangiogenesis. In activated endothelial cells, increased CD147 expression also strongly enhances the angiogenic phenotype by activation of VEGFR2 and PI3K pathway (Figure 1). These findings have made CD147 an important molecule in formation of tumor vessels. Consequently, the development of effective therapeutic interventions targeted to CD147 would provide a novel and potentially powerful alternative to current cancer treatments.
Acknowledgement
This work was supported by Grants from the National Natural Science Foundation of China (No. 81101744), the Fundamental Research Funds for the Central Universities (No. xjj2014144).
References
- Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine 1: 27-30.
- Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249-257.
- Seystahl K, Weller M (2012) Is there a world beyond bevacizumab in targeting angiogenesis in glioblastoma? Expert opinion on investigational drugs 21: 605-617.
- Travasso RD, Poiré EC, Castro M (2016) Correction: Tumor Angiogenesis and Vascular Patterning: A Mathematical Model. PloS one.
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298-307.
- Sennino B, McDonald DM (2012) Controlling escape from angiogenesis inhibitors. Nature Reviews Cancer 12: 699-709.
- Risau W (1997) Mechanisms of angiogenesis. Nature 386: 671-674.
- Risau W, Flamme I (1995) Vasculogenesis. Annual review of cell and developmental biology 11: 73-91.
- Djonov V, Schmid M, Tschanz S (2000) Intussusceptive angiogenesis its role in embryonic vascular network formation. Circulation Research 86: 286-292.
- Dudek AZ, Gupta K, Ramakrishnan S (2012) Tumor angiogenesis. Journal of oncology.
- Kozin SV, Duda DG, Munn LL (2011) Is vasculogenesis crucial for the regrowth of irradiated tumours? Nature Reviews Cancer 11: 532-532.
- Machein MR, Renninger S, Lima-Hahn E (2003) Minor Contribution of Bone Marrow-Derived Endothelial Progenitors to the Vascularization of Murine Gliomas. Brain pathology 13: 582-597.
- Qian CN, Tan MH, Yang JP (2016) Revisiting tumor angiogenesis: vessel co-option, vessel remodeling, and cancer cell-derived vasculature formation. Chinese Journal of Cancer 35: 1.
- Lyden D, Hattori K, Dias S (2001) Impaired recruitment of bone-marrow– derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature medicine 7: 1194-1201.
- Ziegelhoeffer T, Fernandez B, Kostin S, (2004) Bone marrowderived cells do not incorporate into the adult growing vasculature. Circulation research 94: 230-238.
- Hattori K, Dias S, Heissig B (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. The Journal of experimental medicine 193: 1005-1014.
- Khakoo AY, Finkel T (2005) Endothelial progenitor cells. Annu Rev Med 56: 79-101.
- Grunewald M, Avraham I, Dor Y (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124: 175-189.
- Patan S, Munn LL, Jain RK (1996) Intussusceptive microvascular growth in a human colon adenocarcinoma xenograft: a novel mechanism of tumor angiogenesis. Microvascular research 51: 260-272.
- Toole BP (2002) Emmprin (CD147) a cell surface regulator of matrix metalloproteinase production and function. Current topics in developmental biology 54: 371-389.
- Guo H, Li R, Zucker S (2000) EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer research 60: 888-891.
- Chen Z, Yang Z, Mi L (1999) Analysis on the structure and function of hepatoma transfer-associated factor HAb18G. Chinese Journal of Cellular and Molecular Immunology 1: 34-34.
- Biswas C, Zhang Y, DeCastro R (1995) The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer research 55: 434-439.
- Major TC, Liang L, Lu X (2002) Extracellular matrix metalloproteinase inducer (EMMPRIN) is induced upon monocyte differentiation and is expressed in human atheroma. Arterioscler Thromb Vasc Biol 22: 1200-1207.
- Zhu P, Ding J, Zhou J (2005) Expression of CD147 on monocytes/ macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther 7: R1023-1033.
- Sameshima T, Nabeshima K, Toole B P (2003) Correlation of emmprin expression in vascular endothelial cells with blood-brain-barrier function: a study using magnetic resonance imaging enhanced by Gd-DTPA and immunohistochemistry in brain tumors. Virchows Arch 442: 577-584.
- Chen Y, Zhang H, Gou X (2009) Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer Lett 278: 113-121.
- Huet E, Vallee B, Szul D (2008) Extracellular matrix metalloproteinase inducer/CD147 promotes myofibroblast differentiation by inducing alpha-smooth muscle actin expression and collagen gel contraction: implications in tissue remodeling. FASEB J 22: 1144-1154.
- Koch C, Staffler G, Huttinger R (1999) T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int Immunol 11: 777-786.
- Hu J, Dang N, Yao H (2010) Involvement of HAb18G/CD147 in T cell activation and immunological synapse formation. J Cell Mol Med14: 2132-2143.
- Fan Y, Meng S, Wang Y (2011) Visfatin/PBEF/Nampt induces EMMPRIN and MMP-9 production in macrophages via the NAMPTMAPK (p38, ERK1/2)-NF-kappaB signaling pathway. Int J Mol Med 27: 607-615.
- Kim JY, Kim W J, Kim H (2009) The Stimulation of CD147 Induces MMP-9 Expression through ERK and NF-kappaB in Macrophages: Implication for Atherosclerosis. Immune Netw 9: 90-97.
- Woodhead VE, Stonehouse TJ, Binks MH (2000) Novel molecular mechanisms of dendritic cell-induced T cell activation. Int Immunol 12: 1051-1061.
- Schmidt R, Bultmann A, Fischel S (2008) Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ Res 102: 302-309.
- Pennings GJ, Yong AS, Kritharides L (2010) Expression of EMMPRIN (CD147) on circulating platelets in vivo. J Thromb Haemost 8: 472-481.
- Siwik DA, Kuster GM, Brahmbhatt JV (2008) EMMPRIN mediates beta-adrenergic receptor-stimulated matrix metalloproteinase activity in cardiac myocytes. J Mol Cell Cardiol 44: 210-217.
- Huet E, Gabison EE, Mourah S (2008) Role of emmprin/CD147 in tissue remodeling. Connective tissue research 49: 175-179.
- Nabeshima K, Iwasaki H, Koga K (2006) Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathology international 56: 359-367.
- LiYan SZ, Toole BP (2005) Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93: 199-204.
- Muramatsu T, Miyauchi T (2003) Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histology and histopathology 18: 981-987.
- Grass GD, Toole BP (2015) How with Whom and When: An Overview of CD147-mediated Regulatory Networks Influencing Matrix Metalloproteinase Activity. Bioscience reports BSR20150256.
- Sato M, Nakai Y, Nakata W (2013) EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PloS one 8: e74313.
- Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell 21: 309-322.
- Ribatti D, Djonov V (2012) Intussusceptive microvascular growth in tumors. Cancer letters 316: 126-131.
- Rodriguez FJ, Orr BA, Ligon KL (2012) Neoplastic cells are a rare component in human glioblastoma microvasculature. Oncotarget 3: 98.
- Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353-364.
- Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nature Reviews Cancer 8: 592-603.
- Muramatsu T (2015) Basigin (CD147) a multifunctional transmembrane glycoprotein with various binding partners. Journal of biochemistry mvv127.
- Naumov GN, Akslen LA, Folkman J (2006) Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell cycle 5: 1779-1787.
- Norden AD, Drappatz J, Wen PY (2009) Antiangiogenic therapies for high-grade glioma. Nature Reviews Neurology 5: 610-620.
- Egawa N, Koshikawa N, Tomari T (2006) Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. Journal of Biological Chemistry 281: 37576-37585.
- Khayati F, Pérez-Cano L, Maouche K (2015) EMMPRIN/CD147 is a novel coreceptor of VEGFR-2 mediating its activation by VEGF. Oncotarget 6: 9766-9780.
- Koga K, Aoki M, Sameshima T (2011) Synthetic emmprin peptides inhibit tumor cell-fibroblast interaction-stimulated upregulation of MMP-2 and tumor cell invasion. International journal of oncology 39: 657-664.
- Voigt H, Vetter-Kauczok CS, Schrama D (2009) CD147 impacts angiogenesis and metastasis formation. Cancer Invest 27: 329-333.
- Tang Y, Nakada M T, Rafferty P (2006) Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res 4: 371-377.
- Dai L, Bratoeva M, Toole BP (2011) KSHV activation of VEGF secretion and invasion for endothelial cells is mediated through viral upregulation of emmprin-induced signal transduction. Int J Cancer.
- Bougatef F, Quemener C, Kellouche S (2009) EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood 114: 5547-5556.
- Millimaggi D, Mari M, DAscenzo S (2007) Tumor Vesicle-Associated CD147 Modulates the Angiogenic Capability of Endothelial Cells. Neoplasia 9: 349-357.
- Mutin M, Dignat-George F, Sampol J (1997) Immunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface molecules. Tissue antigens 50: 449-458.
- Peters BA, Diaz LA, Polyak K (2009) Contribution of bone marrow– derived endothelial cells to human tumor vasculature. Nature medicine X 11: 261-262.
- Gao D, Nolan D, McDonnell K (2009) Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1796: 33-40.
- Ricci-Vitiani L, Pallini R, Biffoni M (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468: 824-828.
- Wang R, Chadalavada K, Wilshire J (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468: 829-833.
- Chen Y, Gou X, Kong DK (2015) EMMPRIN regulates tumor growth and metastasis by recruiting bone marrow-derived cells through paracrine signaling of SDF-1 and VEGF. Oncotarget 6: 32575-32585.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences