Promising Personalized Anti-Cancer Therapy: the Hidden Molecular Paths for lamin A/C deficiency and Restoration
Capo-chichi D Callinice
DOI10.21767/2573-5365.100011
Capo-chichi D Callinice*
Unit of Biochemistry and Molecular Biology (UBBM), Section of Molecular Biomarkers in Cancer and Nutrition (BMCN), Institute of Biomedical Sciences and Applications (ISBA), Faculty of Sciences and Technology (FAST), University of Abomey Calavi (UAC), Cotonou, Benin
- *Corresponding Author:
- Capo-chichi D Callinice
Unit of Biochemistry and Molecular Biology (UBBM), Section of Molecular Biomarkers in Cancer and Nutrition (BMCN), Institute of Biomedical Sciences and Applications (ISBA), Faculty of Sciences and Technology (FAST), University of Abomey Calavi (UAC), 01BP 526, Cotonou, Benin
Tel: +229 21 36 11 19
E-mail: Callinice.capochichi@gmail.com
Received date: January 21, 2016; Accepted date: February 16, 2015; Published date: February 19, 2016
Citation: Callinice CD. Promising Personalized Anti-Cancer Therapy: the Hidden Molecular Paths for lamin A/C deficiency and Restoration. Cell Mol Med. 2016, 2:1.
Abstract
Carcinogenesis is a complex mechanism that often involves the silencing of cell differentiation and tumor suppressor gene transcripts or proteins while upregulating gene transcripts and proteins involved in cell proliferation and migration. This review focuses on different molecular pathways involved in the suppression of LMNA gene transcripts and lamin A/C proteins known to play important functions in epithelial cells including cell differentiation, gene transcription, cell growth regulation, cell cycle progression and regulation of migration. The restoration of lamin A/C proteins in cancer can only be possible if the mechanism leading to their disappearance is delineated prior to therapy.
Keywords
Lamin A/C; Gene regulation; Post-translational modification; MiRNAs
Introduction
LMNA gene products are lamin A/C proteins which form the nuclear envelop (NE) scaffold in association with several other NE proteins and maintains nuclear structure, organizes chromatins, regulates DNA synthesis and gene transcription, repairs DNA damages, assures proper cell cycle progression, cell migration while maintaining cell differentiated state [1,2]. Cells bearing mutations in LMNA gene or lacking lamin A/C display abnormal nuclear morphology, defective cell cycle kinetics, polyploidy, loss of heterochromatin organization and chromosomal aberration; all of which are hallmark for dedifferentiated and cancer cells [3-5]. The ectopic expression of lamin A/C or the induction of lamin A/C with retinoic acid promotes cell differentiation [6,7]. This makes lamin A/C proteins reliable indicators for differentiated cell [7,8]. Several factors are involved in lamin A/C deficiency from the nuclear envelope and involved phosphorylation, epigenetic modifications (DNA methylation, histone deacetylation), micro RNA, and enzymatic degradation [9-15]. Lamin A/C can be regulated at transcriptional level, translational level and posttranslational levels. In this review we report all the plausible molecular mechanisms of lamin A/C deficiency and the candidate specific drugs that shall restore lamin A/C while killing cancer cells but spare normal cells.
The phosphorylation-dependent lamin A/C degradation induced by P-AKT in cancer is reversible
Lamin A/C proteins are target for serine/threonine (SER/THR) kinases that are overexpressed in cancer cells (e.g. phosphorylated- AKT or p-AKT) and in viruses including human papilloma virus ( HPV), human cytomegalovirus (HCMV) and herpes simplex virus type 1 (HSV-1) protein kinases [9-12]. Human p-AKT1 (protein kinase B, PKB) is responsible for the phosphorylation SER/THR induced degradation of lamin A/C [13]. As matter of fact, p-Akt is viewed as a promising therapeutic target in cancer pathology and therapy; drugs that can inhibit SER/THR kinase activities of p-AKT may also restore lamin A/C [14-16]. P-AKT is viewed as proto-oncogene which functions as a major effector downstream of the Phospho-Inositol-3-kinase (PI3-K) pathways [16]. It was reported that DNA-dependent protein kinase (DNA-PK) which is a PI3-K-like family of kinases, phosphorylates Akt at Ser473 residues in response to genotoxic stimuli associated with double strand breaks DNA [16]. P-Akt is then in a catalytic conformation to phosphorylate specific substrate such as lamin A leading to the cleavage and degradation of phosphorylated lamin A [13,15]. P-Akt-1 phosphorylates lamin A/C at S404, in a canonical Akt consensus motif and participates in cancer cell migration and invasion [16,17]. Overall, p-Akt participates in the post-translational degradation of lamin A/C. The overexpression of p-AKT is observed in cancers including cervical, ovarian and breast cancers [17-20]. As matter of fact, activated PI3K/AKT and MAPK pathways are viewed as potential prognostic markers in cervical cancer progression, in epithelial ovarian cancer and in node-positive triple-negative breast cancer [18-20]. Coincidently the degradation of lamin A/C is also observed in cervical, ovarian and breast cancers [15,21,22]. Drugs that can inhibit the phosphorylation of AKT can reduce cell carcinogenesis and restore lamin A/C [14]. Our experiment with human endometrial cancer cell lines showed that the restoration of lamin A/C can be achieved with Ser/Thr protein kinase inhibitor (staurosporine) as reported earlier [15]. The efficiency of staurosporine as anticancer drugs was reported for cervical cancer and endometrial cancer cell lines [14,15].
The transcriptional regulation of LMNA in cancers through epigenetic modifications is reversible with specific drugs
Epigenetic modifications involve: (i) the methylation of DNA on CpG islands of gene promotor which silences RNA transcription of tumor suppressor gene or (ii) extensive deacetylation of histone near gene promotor which condenses the DNA and makes it refractory to transcription [23-26]. Histone deacetylases (HDACs) are enzymes responsible of the deacetylation of lysine residues of core histones and control chromatin remodeling that represses transcription in compact state of the chromatin associated with deacetylation; the inhibition of HDACs by Histone Deacetylase Inhibitors (HDACIs) restores an open state of chromatin and facilitates gene transcription [23-25]. Epigenetic modifications of tumor suppressor genes including DNA methylation is observed in cervical, ovarian and breast cancers [23-25]. HDACI induced apoptosis of human cervical cancer cells by decreasing DNAmethyltransferase 3B [23]. Natural substances epigallocatechin- 3-gallate and catechin in green tea can reverse the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases (DNMT) and histone deacetylases (HDAC) in human cervical cancer cells [26]. It is now evident that bioactive dietary agents can hamper the process of carcinogenesis by targeting epigenetic alterations including DNA methylation [26, 27]. Green tea can induce the expression of lamin A/C in lung cancer cells [27]. Overall, this data suggests that catechin in green tea might act through the inhibition of DNA methylation and inhibition of HDAC to reinduce lamin A/C in cancer cells [26,27]. The inhibition of DNMT3B and HDAC1 usually reversed the expression of tumor suppressor genes which are silenced by epigenetic modifications but have no effect on genes which are silenced by somatic mutations and polymorphism [28]. There are many DNMT inhibitors from chemical synthesis (5-AZA cytidine: Vidaza 5-AZA 2’-deoxycytidine: Decitabine, Dacogen) or from natural sources (polyphenols, flavonoids, antraquinones etc.) which are extracted from plants [29]. Resveratrol is also a natural polyphenol with plethora of biological activities including DNMT inhibition enhanced with resveratrol-salicylate derivatives which have better DNMT3 inhibitory activity by binding with DNMT3A and DNMT3B enzymes [30]. Thus the conjugation of Resveratrol to aspirin exerts better chemopreventive effects than Resveratrol alone [30]. All DNMT inhibitors stated above are summarized in Table 1. HDACIs usually are a natural defense substance against exogenous macromolecules or microorganisms (e.g. viruses, bacteria); the HDACI trichostatin A (TSA) also known as an organic antifungal antibiotic is produced by Streptomyces hygroscopicu and has anti-tumor activity [31,32]. TSA is a potent anti-cancer drug for cervical and endometrial cancer cells but all the molecular pathways were not elucidated [15,23]. The use of TSA restored lamin A/C expression and induced cell apoptosis but is not yet used for human therapy [15,23]. TSA acts against HDAC class I and II; two HDACIs such as vorinostat (SAHA) and romidepsin, were approved by Food and Drug Administration (FDA) agency to be used against cancer [33]. HDACIs are classified in different groups as specified in Table 1: TSA, and vorinostat belong to the group of hydroxamic acids; valproate and butyrate belong to the group of carboxylic acids; entinostat and mocetinostat belong to the group of aminobenzamides; apicidin and romidepsin are in the group of cyclic peptides; trapoxins are in the group of epoxyketones [33]. Many more HDACIs are being studied to be used as anticancer drugs. Efficient HDACI should induce tumor cell growth arrest, cell differentiation or apoptosis [14,15,23]. Due to the variety of HDAC, the target specificity of HDACIs and the requirement for specific and selective inhibition of HDACs, we suggest that the bioactivity of each HDACIs should be tested in vitro on 3-D culture of cancer-derived cells; followed by biochemical analyses to assess the histone acetylation levels by chromatin immunoprecipitation (Chip) assay associated to immuno blotting with anti-acetyl histone antibody, or direct analysis of acetyl-histone in cells by immunohistochemistry or immunofluorescence before selecting the potent therapeutic HDACI for each case. Our report demonstrated that lamin A/C proteins may be part of the molecular mechanism that establishes the anticancer potency of HDACI and Ser/Thr protein kinase inhibitors. Thus, the potency of anti-cancer therapy could be evaluated by the efficiency of restoration of lamin A/C [15] in cancer cells that had lost the expression of these proteins. The new generations of drugs that are being developed to reverse epigenetic modifications in cancer are hybrid molecules to target HDACs and other oncogenic proteins and their pathways; the ester of HDACI contains an esterase sensitive chemical motif which can be hydrolyzed by white blood cells (monocyte and macrophage) before trapping of the active drug inside cancer cells [33]. All HDACIs stated above are summarized in (Table 1).
| Drugs A | Drugs B | Drugs C | |
|---|---|---|---|
| DNMT I | HDAC I | miR inducers | Protein kinase inhibitors |
| Chemical synthesis 5-AZA cytidine: Vidaza 5-AZA 2’-deoxycytidine: Decitabine, Dacogen Natural sources polyphenols, flavonoids, antraquinones Polyphenol and derivatives: Resveratrol Resveratrol-salicylate Polyphenol in Green Tea extracts: Catechins, Epigallocatechin-3-gallate (EGCG) [26-30] |
Hydroxamic acids: Trichostatin A and vorinostat (SAHA) Carboxylic acids: valproate and butyrate Aminobenzamides: entinostat and mocetinostat Cyclic peptides: apicidin and romidepsin Epoxyketones: trapoxins[14,15,23,33] |
Curcumin (diferuloylmethane): induces miR7:inhibitor of EGFR/ PI3K/AKT pathways [50-53] | Staurosporine: Protein kinase C inhibitor, SER/THR inhibitor Rapamycin, ridaforolimus, curcumin: PI3K/AKT/mTOR pathway inhibitors [48,49] |
Table 1: Summary of potent anti-cancer drugs that can restore the expression of lamin A/C.
The post-transcriptional regulation of lamin A/C mRNA by anti-sense non-coding small RNAs (miRNAs)
The expression of LMNA gene can also be regulated at the post transcriptional level by non-protein-coding endogenous anti sense small RNA molecules, also known as microRNAs (miRNAs or miR), which acts as repressors of gene expression by pairing the 3’-UTR of mRNAs, thereby causing the degradation of lamin A/C mRNA and inhibition of protein translation by ribosomes [34]. The maturation of pre-lamin A into lamin A depends on ZMPSTE24 enzyme which can be suppressed by the direct binding of miR-141-3p to the 3'UTR of ZMPSTE24 transcripts to impair the post-translational maturation of lamin A and generate numerous pathologies linked to the absence of functional lamin A including premature aging or cancers [35]. In addition to their structural role, lamin A/C proteins also take part in gene transcription regulation in such extend that the absence of functional lamin A/C is associated to an over-expression of miRNA and gene dysregulation [34,35]. Studies showed that the up-regulation of MiRNA-205 in ovarian cancer cells exposed to VEGF suppressed cell growth regulation genes including LMNA (lamin A/C proteins) and promotes the invasion and proliferation of ovarian cancer cells [36]. A genome-wide expression profiling revealed the dysregulation in various mRNA transcripts including those in MAPK, transforming growth factor-β (TGF β) and Wnt signaling pathways as well as dysregulation of mRNA transcripts of genes involved in skeletal muscle differentiation and proliferation along with renal cell carcinoma, basal cell carcinoma and prostate cancer genes [34]. Increased TGFβ-signaling activity prevents skeletal muscle regeneration but can promote cancer [34]. Lamin A/C mutants affect RNA-Polymerase II (RNA-Pol II) shown to be involved in miRNA transcription [37,38].
The transcriptional regulation of LMNA gene is associated to the transcriptional regulation of GATA proteins
The transcription factor GATA6 is an indirect regulator of lamin A/C and silencing of GATA6 and lamin A/C had been reported in ovarian and cervical cancers [39,40]. Both GATA6 and lamin A/C are essential for cell differentiation and in maintaining a differentiated state of epithelial cell; their absence is associated to cell dedifferentiation and carcinogenesis [39,40]. The downregulation of GATA6 was shown to be associated to overexpression of miRNA-363 and miRNA-1274b [41]. LMNA transcript is also target for miR-363 and 1274b [42]. Overall, miR-363 and miRNA-1274b may sequentially downregulate both GATA6 and LMNA prior to ovarian and cervical carcinogenesis [15,21]. In Breast epithelial cell GATA3 and lamin A/C have roles in maintaining cell differentiated state [22,43]. Both GATA3 and LMNA may be target for miRNA-205 and miRNA-1274b in breast cancer [42,43]. MiRNA profiling in cervical cancer tissues has revealed the presence of many miRNAs with multiple pathway regulation functions [44]. MiRNA-21 is shown to be frequently up-regulated in breast, gastric, colon, lung, pancreatic and ovarian cancers [44,45]. Transcription factors GATA6 and GATA3 are both regulated by miRNA-302 cluster, and overall survival was shorter in patients with miRNA-302-positive cancer cells [45-47]. The implication of miRNA in carcinogenesis is not fully elucidated but transcription factors are targeted by specific miRNA overexpressed in cancers and more studies are needed to develop drugs that regulate miRNAs as some miRNA are pro-oncogenic while others are anti-oncogenic [41-47]. Pro-oncogenic miRNAs target cell differentiation and tumor-suppressor gene transcripts while anti-oncogenic-miRs target cell proliferation and migration gene transcripts [41-47].
Summary of the different mechanisms that may be involved in the silencing of lamin A/C prior to carcinogenesis
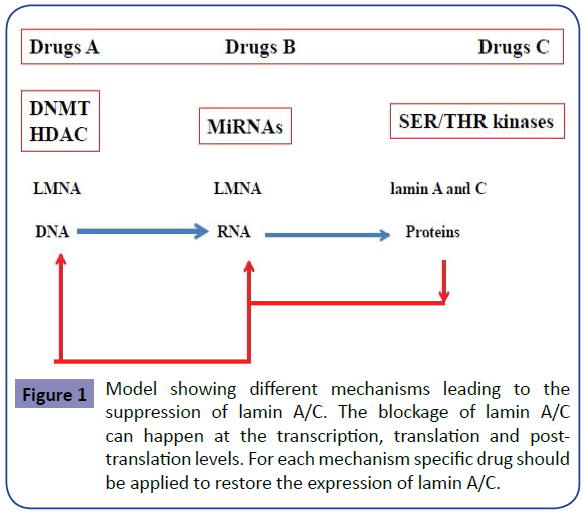
The model showing different mechanisms leading to the suppression of lamin A/C is shown in Figure 1. The blockage of lamin A/C can happen at 3 different levels with respect to the central dogma of molecular biology: (i) the transcription level, (ii) the translation level and (iii) the post-translation level. For each mechanism specific drug should be applied to restore the expression of lamin A/C and to cure the disease associated to it. Therefore personalized therapy should always be requested to restore the expression of lamin A/C according to pathway affected and the corresponding drugs shown respectively in Figure 1 and Table 1.
Overall promising treatments
Analogues of small molecule inhibitors targeting simultaneously histone deacetylation and phosphorylation pathways like HDAC, PI3K/AKT and RAF-MEK-MAPK pathways in human cancer cell lines and xenografts should be more investigated to overcome cancer diseases [48]. A combination of vorinostat (HDAC inhibitor) with rapamycin (PI3K/AKT/mTOR pathway inhibitors) or LY294002 should also be considered for cancer treatment [48,49]. Curcumin (diferuloylmethane), a natural polyphenol of curcuma species, inhibits cell growth and invasion through up-regulation of miR- 7 in pancreatic cancer cells [50,51]. MiR-7 is a potential tumor suppressor that acts by targeting multiple oncogenes related pathways including PI3/AKT and may serve as a novel therapeutic target for cancer [50,51]. The anti-tumor effect of curcumin was reported for numerous cancers including breast cancer and leads to AKT degradation, suppression of proliferation and migration in cancer cells associated to autophagy [52]. Curcumin is also a potent inhibitor of protein kinase C (PKC), NF(nucleor factor) kappa B (NFκB), and, MAPKs, ERK, ELK, PI3K, Akt, CDKs and iNOS [53]. This multiple oncogene inhibition function of curcumin might be possible through the induction of miRs including miR-7 [50,51]. Overall, curcumin inhibits cell growth and invasion through the up-regulation of miR-7 and downregulation of miR21 in cancer cells to disrupt multiple oncogenic pathways [50-54]. Curcumin is stated in Table 1 as one of miRNA anti-cancer therapy drugs.
Conclusion
Due to the plethora of mechanisms leading to the down regulation of lamin A/C in cancers, it is crucial to proceed to in vitro analysis to delineate potent drugs for the restoration of lamin A/C and regulation of cell growth in cancer derived cells before using them to cure cancers in human. Therefore, personalized molecular medicine is more appropriate to treat cancer and avoid drug resistance and toxicity linked to no-specific chemotherapy.
References
- Bridger JM, Foeger N, Kill IR, Herrmann H (2007) The nuclear lamina Both a structural framework and a platform for genome organization. FEBS J 274: 1354-1361.
- Gant TM, Wilson KL (1997) Nuclear assembly. Annu Rev Cell Dev Biol 13: 669-695.
- Corso C, Parry EM, Faragher RG, Seager A, Green MH,et al. (2005) Molecular cytogenetic insights into the ageing syndrome Hutchinson-Gilford Progeria (HGPS). Cytogenet Genome Res 111:27-33.
- Willis ND, Cox TR, Rahman-Casañs SF, Smits K, Przyborski SA,et al. (2008)Lamin A/C is a risk biomarker in colorectal cancer. PLoS One 3:e2988.
- Venables RS, McLean S, Luny D, Moteleb E, Morley S,et al.(2001) Expression of individual lamins in basal cell carcinomas of the skin. Br J Cancer 84:512-519.
- Lourim D,Limm JJ-C (1992) Expression of wild-type and nuclear localization deficient laminA in chick myoblasts. J Cell Sci 123: 501–512.
- Smith ER, Zhang XY, Capo-Chichi CD, Chen X, Xu XX (2011) Increased expression of Syne1/nesprin-1 facilitates nuclear envelope structure changes in embryonic stem cell differentiation. Dev Dyn 240:2245-2255.
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB (2006)Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24:177-185.
- Kivi N, Greco D, Auvinen P, Auvinen E (2008) Genes involved in cell adhesion, cell motility and mitogenic signaling are altered due to HPV 16 E5 protein expression. Oncogene 17:2532-41.
- Sharma M, Bender BJ, Kamil JP, Lye MF, Pesola JM, et al. (2015) Human cytomegalovirus UL97 phosphorylates the viral nuclear egress complex. J Virol 89:523-534.
- Mou F, Forest T, Baines JD(2007) US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol 81:6459-6470.
- Cano Monreal GL, Wylie KM, Cao F, Tavis JE, Morrison LA (2009) Herpes simplex virus 2 UL13 protein kinase disrupts nuclear lamins. Virology 392:137-147.
- Naeem AS, Zhu Y, Di WL, Marmiroli S, OShaughnessy RF (2015) AKT1-mediated Lamin A/C degradation is required for nuclear degradation and normal epidermal terminal differentiation.Cell Death Differ 22:2123-2132.
- Bernard B, Prétet JL, Charlot JF, MouginC (2003) Human papillomaviruses type 16+ and 18+ cervical carcinoma cells are sensitive to staurosporine-mediated apoptosis. Biol Cell 95: 17-26.
- Capo-Chichi CD, Aguida B, Chabi NW, Cai QK, Offrin G (2015)Lamin A/C deficiency is an independent risk factor for cervical cancer. Cell Oncol (Dordr).
- Toker A, Marmiroli S (2014) Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul55: 28-38.
- Cenni V, Bertacchini J, Beretti F, Lattanzi G, Bavelloni A,et al. (2008) Lamin A Ser 404 is a nuclear target of Akt phosphorylation in C2C12 cells. J Proteome Res 7:4727-4735.
- Choi SK, Hong YO, Lee WM, Kim EK, Joo JE, et al. (2015) Overexpression of PI3K-p110α in the progression of uterine cervical neoplasia and its correlation with pAkt and DJ-1. Eur J Gynaecol Oncol 36:389-393.
- Cai J, Xu L, Tang H, Yang Q, Yi X, et al. (2014) The role of the PTEN/PI3K/Akt pathway on prognosis in epithelial ovarian cancer: a meta-analysis. Oncologist 19: 528-535.
- Hashimoto K, Tsuda H, Koizumi F, Shimizu C, Yonemori K, et al. (2014) Activated PI3K/AKT and MAPK pathways are potential good prognostic markers in node-positive, triple-negative breast cancer. Ann Oncol 25:1973-1979.
- Capo-chichi CD, Cai KQ, Simpkins F, Ganjei-Azar P, Godwin AK, et al. (2011) Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer. BMC Med 26: 28.
- Capo-chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, et al. (2011) Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chin J Cancer 30:415-425.
- Liu N, Zhao LJ, Li XP, Wang JL, Chai GL, et al. (2012) Histone deacetylase inhibitors inducing human cervical cancer cell apoptosis by decreasing DNA-methyltransferase 3B. Chin Med J (Engl) 125:3273-3278.
- Pchejetski D, Alfraidi A, Sacco K, Alshaker H, Muhammad A, et al. (2015) Histone deacetylases as new therapy targets for platinum-resistant epithelial ovarian cancer. J Cancer Res Clin Oncol.
- Rauscher GH, Kresovich JK, Poulin M, Yan L, Macias V, et al. (2015) Exploring DNA methylation changes in promoter, intragenic, and intergenic regions as early and late events in breast cancer formation. BMC Cancer 15: 816.
- Khan MA, Hussain A, Sundaram MK, Alalami U, Gunasekera D,et al. (2015) (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol Rep 33:1976-1984.
- Lu QY, Yang Y, Jin YS, Zhang ZF, Heber D, et al. (2009)Dubinett SM, Sondej MA, Loo JA, Rao JY. Effects of green tea extract on lung cancer A549 cells: proteomic identification of proteins associated with cell migration. Proteomics 9: 757–767.
- LaBarge MA, Mora-Blanco EL, Samson S, Miyano M(2015) Breast Cancer beyond the Age of Mutation. Gerontology.
- Zwergel C, Valente S, Mai A (2016) DNA Methyltransferases Inhibitors from Natural Sources. Curr Top Med Chem 16:680-696.
- Aldawsari FS, Aguayo-Ortiz R, Kapilashrami K, Yoo J, Luo M,et al. (2015) Resveratrol-salicylate derivatives as selective DNMT3 inhibitors and anticancer agents. J Enzyme Inhib Med Chem 29:1-9.
- Shankar S, Srivastava RK (2008) Histone deacetylase inhibitors: mechanisms and clinical significance in cancer: HDAC inhibitor-induced apoptosis. Adv Exp Med Biol 615:261-298.
- Lamoth F, Juvvadi PR, Steinbach WJ(2015) Histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. Front Microbiol 6: 96.
- West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124: 30–39.
- Sylvius N, Bonne G, Straatman K, Reddy T, Gant TW, et al.(2011) MicroRNA expression profiling in patients with lamin A/C-associated muscular dystrophy.FASEB J 25:3966-3978.
- Yu KR, Lee S, Jung JW, Hong IS, Kim HS, et al. (2013) MicroRNA-141-3p plays a role in human mesenchymal stem cell aging by directly targeting ZMPSTE24. J Cell Sci 126:5422-5431.
- Li J, Li L, Li Z, Gong G, Chen P,et al.(2015)The role of miR-205 in the VEGF-mediated promotion of human ovarian cancer cell invasion.Gynecol Oncol137:125-133.
- Spann TP, Goldman AE, Wang C, Huang S, Goldman R D (2002) Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol 156: 603-608.
- Lee Y, Kim M, Han J, Yeom KH, Lee S, et al. (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060.
- Capo-chichi CD, Cai KQ, Testa JR, Godwin AK, Xu XX (2009) Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer. Mol Cell Biol29:4766-4777.
- Capo-chichi CD, Aguida B, Cai QK, Offrin G, Agossou VK, et al. (2015) The Deficiency of Nuclear Proteins GATA6 and Lamin A/C as Prognostic Factor for Cervical Neoplasia.American Journal of Cancer Prevention 3: 109-116.
- Tsuji S, Kawasaki Y, Furukawa S, Taniue K, Hayashi T, et al.(2014)The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. NatCommun 5:3150.
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, et al.(2005) Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37: 766-770.
- Mehra R, Varambally S, Ding L, Shen R, Sabel MS, et al. (2005) Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res 65:11259-11264.
- Lui WO, Pourmand N, Patterson BK, Fire A (2007) Patterns of known and novel small RNAs in human cervical cancer. Cancer Res 67: 6031-6043.
- Esquela-Kerscher A, Slack FJ(2006) Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer 6:259-269.
- Volinia S, Nuovo G, Drusco A, Costinean S, Abujarour R, et al.(2014) Pluripotent stem cell miRNAs and metastasis in invasive breast cancer. J Natl Cancer Inst 106: 324.
- Murray MJ, Saini HK, van Dongen S, Palmer RD, Muralidhar B, et al. (2010) The two most common histological subtypes of malignant germ cell tumour are distinguished by global microRNA profiles, associated with differential transcription factor expression. Mol Cancer 9: 290.
- Qian C, Lai CJ, Bao R, Wang DG, Wang J, et al. (2012) Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clinical CancerResearch 18: 4104-4113.
- Quan P, Moinfar F, Kufferath I, Absenger M, Kueznik T, et al. (2014) Effects of targeting endometrial stromal sarcoma cells via histone deacetylase and PI3K/AKT/mTOR signaling. Anticancer Research 34: 2883–2897.
- Ma J, Fang B, Zeng F, Pang H, Zhang J, et al. (2014) Curcumin inhibits cell growth and invasion through up-regulation of miR-7 in pancreatic cancer cells. Toxicol Lett 231: 82-91.
- Liu Z, Jiang Z, Huang J, Huang S, Li Y, et al. (2014) miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int J Oncol 4:1571-1580.
- Guan F, Ding Y, Zhang Y, Zhou Y, Li M, et al. (2016) Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS One 11: e0146553.
- Lin JK (2004) Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res 27:683-692.
- Si ML, Zhu S, Wu H, Lu Z, Wu F, et al. (2007) miR-21-mediated tumor growth. Oncogene 26:2799-2803.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences