Selective HDAC6 Inhibition Corrects Aberrant B Cell Development in the Bone Marrow of NZB/W F1 Mice
Miranda D Vieson, Xin M Luo, Song Li, Alexander M Gojmerac, Adrian Castaneda and Christopher M Reilly
DOI10.21767/2573-5365.100020
Miranda D Vieson1, Xin M Luo1, Song Li2, Alexander M Gojmerac3, Adrian Castaneda1 and Christopher M Reilly1,4*
1Department of Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine, Blacksburg, Virginia
2College of Agricultural and Life Sciences, Virginia Tech, Blacksburg, Virginia
3Department of Biological Sciences, Virginia Tech, Blacksburg, Virginia
4Edward Via College of Osteopathic Medicine, Blacksburg, Virginia
- *Corresponding Author:
- Christopher M Reilly
Department of Biomedical Sciences and Pathobiology
Virginia-Maryland College of Veterinary Medicine
Blacksburg, 24061, Virginia
Tel: 540-231-1744
E-mail: chreilly@vcom.vt.edu
Received Date: August 31, 2016; Accepted Date: September 27, 2016; Published Date: October 03, 2016
Citation: Vieson MD, Luo XM, Li S, et al. Selective HDAC6 inhibition corrects aberrant B cell development in the bone marrow of NZB/W F1 mice. Cell Mol Med. 2016, 2:3.
Abstract
B cell development in the bone marrow is highly complex and includes vital regulatory checkpoints to maintain central tolerance. Defects in central tolerance are implicated in systemic lupus erythematosus (SLE) and aberrant B cell development has been reported in NZB/W mice. We hypothesized that altered B cell development in the bone marrow of lupus-prone NZB/W mice would be corrected after HDAC6 inhibition. B cell development was evaluated by flow cytometric analysis of Hardy fractions from bone marrow cells of NZB/W mice treated with an HDAC6 inhibitor or vehicle control. Additionally, deep sequence analysis of RNA from the bone marrow was utilized to identify potential targets of HDAC6. As NZB/W mice aged, there was an apparent shift in later stages of B cell development suggesting accelerated progression through maturation and potential to bypass key regulatory checkpoints. After HDAC6 inhibition, the alteration was corrected and RNAseq analysis revealed differential expression of 849 genes in the bone marrow. We focused on 6 genes related to B cell development and differentiation (ccr9, spib, pou2af1, nfil3, cebpb, and lgals1) and found that HDAC6 appears to have the most impact on expression of spiB, an early regulator of B cell development, and pou2af1, a regulator during later stages of B cell development. We conclude that HDAC6 inhibition helps correct aberrant B cell development and differentiation in the bone marrow of lupus-prone NZB/W mice. These results also identified new potential targets for HDAC6 regulation within the bone marrow, particularly spiB and pou2af1.
Keywords
B cell development; Bone marrow; Histone deacetylase 6; RNA seq; Systemic lupus erythematosus
Introduction
Mechanisms regulating the development of autoreactive B cells in the bone marrow are referred to as central tolerance. The three main mechanisms of self-tolerance are deletion by apoptosis, secondary recombination events of the B cell receptor (BCR), and energy. Defects in any of these mechanisms may result in the production of autoreactive B cells that enter circulation and contribute to autoimmune diseases like (SLE) [1]. Central tolerance as a factor inducing autoimmunity was demonstrated after transplantation of bone marrow cells from New Zealand black (NZB) mice into irradiated DBA/2 mice incited manifestations of autoimmune disease [2]. In regard to human SLE, there is a high frequency of auto-reactivity in new emigrant and mature naïve B cells in SLE patients as well as BCR signaling abnormalities and altered receptor editing by secondary recombination in SLE B cells [3].
B cells originate from pluripotent hematopoietic stem cells (HSCs) within the bone marrow, a subset of which develop into common lymphoid progenitor cells (CLPs) that can further develop into early B lineage cells [4]. Beyond this point, development and maturation of B cells progresses through phenotypic stages classified by 3 systems of nomenclature (Philadelphia, Basel, and Hardy) [5,6], which are summarized in Figure 1. Within the Hardy classification scheme, B cells first progress through pro B cell stages A, B, C, then C’ followed by progression through pre B cell stages D, E, then F. At least two checkpoints have been postulated during B cell development in the bone marrow where central tolerance likely regulates auto-reactive B cells. The first checkpoint occurs within the C and C’ Fractions. At this point in development, it appears that proliferation of large pre B cells is over least twice the number of cells that progress to the small pre B cell population in its steady state, suggesting that over half the large B cells are prevented from differentiating further and subsequently deleted [7,8]. A second checkpoint occurs between the D and E fractions. At this stage of development, many early immature B cells express auto-reactive antibodies including antinuclear antibodies (ANAs) and are subsequently removed before development into immature B cells (Fraction E) [9].
Figure 1: B cell developmental stages in the bone marrow. The first phenotypic stage of B cell development is composed of cells that are B220+CD43+CD24-BP1-, which are included in Fraction A and referred to as Pre-Pro B cells (Philadelphia) or Pro- B cells (Basel). The Basel classification scheme is primarily based on the status of Ig gene rearrangements; cells in the germline configuration are referred to as Pro-B cells. Cells in Fractions B (B220+CD43+CD24+BP-1-) and C (B220+CD43+CD24lowBP-1+) are grouped together in the Philadelphia and Basel systems as Pro-B cells or Pre-BI cells, respectively. At this stage, Pro-B cells are defined as those expressing a unique intranuclear enzyme, TdT, that is active during VH-gene rearrangement. Pre-BI cells are those that have undergone partial Ig H-gene rearrangements of diversity (D) and joining (J) segments. Cells then progress into the C’ fraction where cells are B220+CD43+CD24highBP-1+ and now referred to as early pre-B cells in the Philadelphia system and pre-BII cells in the basal system. Pre-B cells are phenotypically defined by the expression Ig μ H chains within the cytoplasm (cμ) and then further subdivided into two populations based on their size and mitotic activity. The large and mitotically active (early Pre-B) cells are included in the C’ fraction. These cells also have a fully rearranged H-chain locus at this point, and thus referred to as large Pre-BII cells. The small, non-dividing (late Pre-B) cells are included in the next developmental Hardy fraction, D, where cells are B220lowCD43-IgM-IgD-. At this stage, cells now have fully rearranged H- and L-chain loci and may also be referred to as small pre-BII cells (Basal). Lastly, the cells go through fractions E then F. Cells in Fraction E, or new B cells (Philadelphia) begin expressing IgM on the cell surface (B220lowCD43-IgM+IgD-), which is a universally accepted criterion of immature B cells. Cells in fraction F express both IgM and IgD (B220highCD43-IgM+IgD+) and can also be referred to as mature B cells. †=Total percent of cells in within Hardy fractions from the bone marrow of BALB/c mice (3), *=Regulatory checkpoint #1, **=Regulatory checkpoint #2.
Our laboratory has recently documented alterations in proportions of B cells within Hardy fractions in the bone marrow of diseased NZB/W mice. This suggests abnormalities in B cell development contribute to disease progression. Specifically, we noted a significantly decreased percentage of cells in the C, C’, D and E fractions, and a significant increase in cells within the F fraction in 38 week-old (diseased) mice compared to pre-diseased (8 week-old) mice. These results led us to believe that B cells may progress through developmental stages more rapidly and bypass central tolerance checkpoints allowing increased numbers of auto-reactive B cells to be released into circulation and contribute to lupus disease [10].
Histone deacetylase (HDAC) 6 is a class IIb HDAC enzyme that predominantly localizes in the cytoplasm and modulates the acetylation status and subsequent function of multiple proteins that play a role in SLE [11]. Expression and activity of HDAC6 is increased in B cells from the bone marrow of diseased lupus-prone mice [12]. Furthermore, selective HDAC6 inhibition in NZB/W mice decreased lupus nephritis and increased proportions of bone marrow B cells within the C, D, and E fractions with a corresponding decrease in the F fraction [10].
In our current studies, we sought to understand further the progression of B cells through developmental fractions in the bone marrow of NZB/W mice as they age and develop disease. Additionally, we investigated the role of HDAC6 in contributing to altering the proportions of B cells in bone marrow developmental fractions.
Materials and Methods
Mice
Female NZB/W F1 mice were purchased from Jackson laboratories (Bar Harbor, ME, USA). All mice were used in accordance with the institutional animal care and use committee (IACUC) after protocol approval by the IACUC of Virginia Tech University and housed in the animal facility at the Virginia-Maryland College of Veterinary Medicine (Blacksburg, VA, USA).
In vivo treatments with HDAC6 inhibitors
ACY-1083 and ACY-738, selective histone deacetylase (HDAC) 6 inhibitors, were courtesy of a generous donation from Acetylon pharmaceuticals (Boston, MA, USA) for use in all studies. ACY-1083 was dissolved in 0.05% hydroxy-propylmethyl cellulose (HPMC, Sigma, St. Louis, MO, USA) and ACY-738 was dissolved in dimethyl sulfoxide (DMSO). All mice were injected intraperitoneally (IP) 5 days/week with a 50 μL volume of their respective treatments. Beginning at 21-weeksof- age, ten mice were included in each of 5 treatment groups: 1) vehicle control (HPMC), 2) 0.3 mg/kg ACY-1083, 3) 1 mg/kg ACY-1083, 4) 3 mg/kg ACY-1083 and 5) 2 mg/kg dexamethasone (TCI America, Portland, OR, USA). Treatments in these groups continued until euthanasia during late stage clinical disease at 34 weeks-of-age. A separate group of 10 mice were not treated until 35 weeks-of-age at which point they were separated into 2 treatment groups and received either 40 mg/kg ACY-738 or vehicle control (DMSO) treatments for 2 weeks followed by euthanasia. In a pilot study, ACY-738 was formulated in rodent chow at 100 mg/kg and fed to 10 mice ad libitum. For control groups, 10 mice were fed standard rodent chow (negative control) and 10 mice were treated with dexamethasone (2 mg/kg) IP (positive control). Treatment was initiated at 16 weeks-of-age and continued until euthanasia at 34 weeks-of-age.
Flow cytometric analysis
After euthanasia, femurs and tibias were isolated, then bone marrows were flushed out and collected with cold PBS containing 1% BSA to create a single cell suspension. The cells were washed with cold DPBS followed by staining of cell surface antigens with directly conjugated fluorescent-labeled murine monoclonal antibodies (eBiosciences, San Diego, CA, USA). Cells were sorted by a FACS Aria 1 flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo software (Tree Star, Ashland, OR, USA). Cells from the bone marrow were first gated by CD43-fluorescein isothiocyanate (FITC) and B220/CD45R-allophycocyanin (APC) staining into Pro B cell (B220+CD43+) and Pre B cell (B220+CD43-) fractions. The Pro B cell fractions were further gated by CD24-eFlour450 and BP1-phycoerythrin (PE) staining into Fractions A (CD24-BP1-), B (CD24+BP1-), C (CD24loBP1+), and C’ (CD24hiBP1+). The Pre B cell fraction was further gated by IgM-eFluor450 and B220- APC staining into Fractions D (IgM-B220lo), E (IgM+B220lo), and F (IgM+B220hi).
RNA seq and analysis
Total RNA was isolated from primary bone marrow cells using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA) per manufacturer’s instructions. Total RNA was sent to Beckman Coulter (Danvers, MA, USA) for 2 × 100 bp paired-end Illumina RNA sequencing with an average of 40 million reads per sample. Sequencing data (FASTQ files) was trimmed for both adaptor sequences and quality using a combination of ea-utils and Btrim [13,14]. Sequencing reads were then aligned to the genome (Ensembl.org 38.74) using Bowtie2/Tophat2 [15,16] and counted via HTSeq [17]. Following successful alignment and counting, statistical analysis of mRNA differential expression was determined using the Benjamini-Hochberg corrected Wald Test (FDR<0.1) in the R-package DESeq2 (R version 3.1.1, DESeq2 version 1.4.5) [18].
Real-time PCR
Reverse transcription was first performed on isolated total RNA using a high-capacity cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA) per manufacturer’s instructions. Real-time PCR was then performed utilizing the TaqMan Gene Expression Assay system (Applied Biosystems, Foster City, CA, USA) per manufacturer’s instructions. Expression levels were normalized to an endogenous control, GAPDH. Data are reported as relative expression levels compared to the vehicle control group by calculating with formula 2-ΔΔCt (Livak method).
Renal histopathology
At the time of euthanasia, both kidneys were removed. One kidney was fixed in 10% neutral buffered formalin for 24 hours, then routinely processed, embedded in paraffin, sectioned at 4 μm to 5 μm, and stained with periodic acid-Schiff (PAS). Kidney sections were scored (0-4) for glomerular proliferation, inflammation, crescent formation, necrosis, and fibrosis by a board certified veterinary pathologist in a blinded manner.
Statistics
Statistical analysis was performed using GraphPad Prism Version 6 software (La Jolla, CA, USA). Differences among groups with more than two conditions were analyzed using one-way ANOVA followed by further analysis using Tukey’s multiple comparison tests. Differences between two means were assessed with unpaired two-tailed t-tests. A p-value <0.05 was considered statistically significant.
Results and Discussion
Decreases in percentages of developing B cells in the bone marrow occur mostly within Hardy fractions B and D with a concurrent increase in fraction F as NZB/W mice age
Hardy fractions organize B cells into sequential stages of development and differentiation based on their phenotypic expression of select progenitor and B cell markers [5]. Previous studies have shown aberrant development and differentiation of B cells in the bone marrow of NZB/W mice [10], although the point in disease progression where abnormalities in the bone marrow arise is uncertain. Therefore, we sought to characterize further B cell development and differentiation in the bone marrow by investigating the percentage of cells within Hardy fractions in female NZB/W mice at 23, 27, 32, and 35 weeks-of-age (Figure 2). The sorting and gating scheme for Hardy fractions (5) is characterized (Figure 2A) by first gating into pro B cell (B220+CD43+) and pre B cell (B220+CD43-) fractions. The pro B cell fractions were further gated into fractions A (CD24-BP1-), B (CD24+BP1-), C (CD24loBP1+), and C’ (CD24hiBP1+). The pre B cell fraction was further gated into fractions D (IgM-B220lo), E (IgM+B220lo), and F (IgM+B220hi). Data throughout is presented as total percentages of all nucleated cells in the bone marrow.
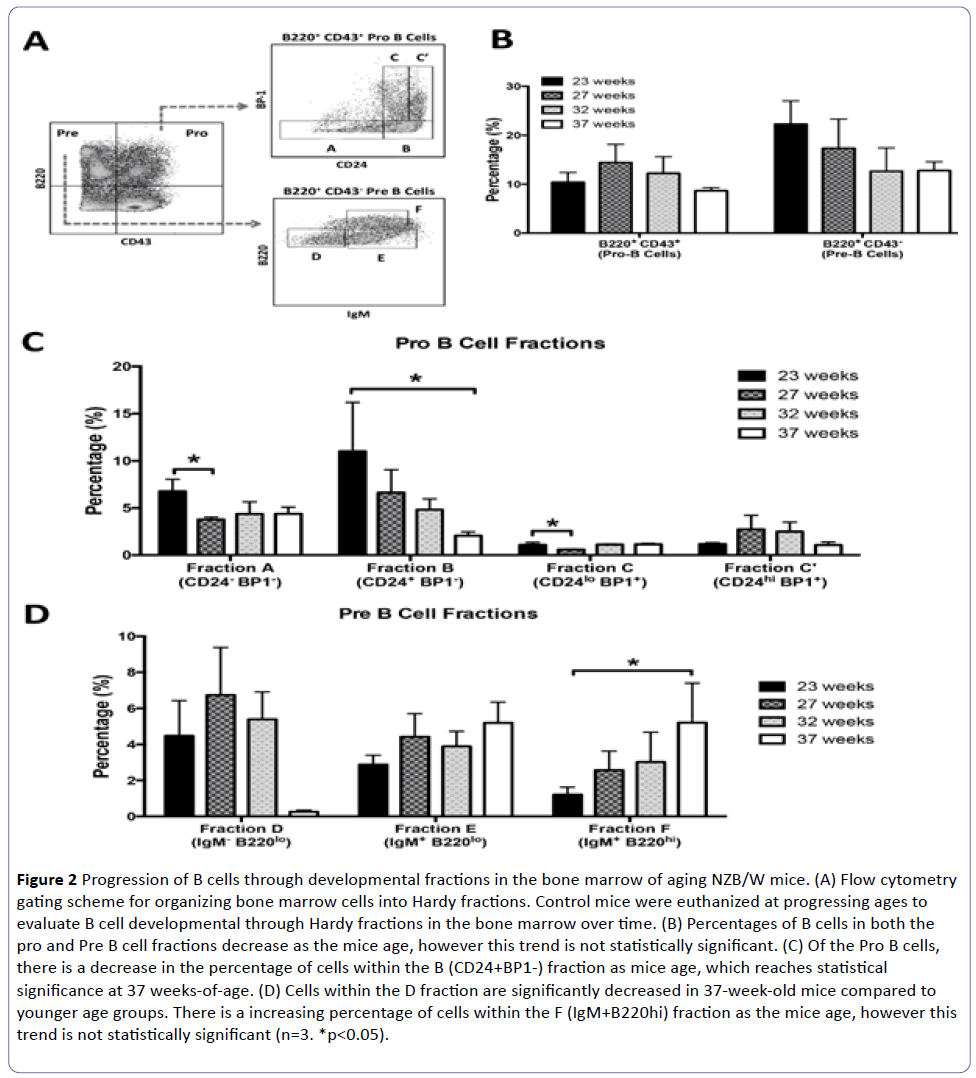
Figure 2: Progression of B cells through developmental fractions in the bone marrow of aging NZB/W mice. (A) Flow cytometry gating scheme for organizing bone marrow cells into Hardy fractions. Control mice were euthanized at progressing ages to evaluate B cell developmental through Hardy fractions in the bone marrow over time. (B) Percentages of B cells in both the pro and Pre B cell fractions decrease as the mice age, however this trend is not statistically significant. (C) Of the Pro B cells, there is a decrease in the percentage of cells within the B (CD24+BP1-) fraction as mice age, which reaches statistical significance at 37 weeks-of-age. (D) Cells within the D fraction are significantly decreased in 37-week-old mice compared to younger age groups. There is a increasing percentage of cells within the F (IgM+B220hi) fraction as the mice age, however this trend is not statistically significant (n=3. *p<0.05).
There was a decrease in the percentage of pro and pre B cells within the bone marrow as the mice aged, although this was not statistically significant (Figure 2B). The percentage of cells within the B fraction progressively decreased in NZB/W mice as they aged and reached statistical significance at 37 weeks-of-age (Figure 2C). A significant decrease in the A and C fractions was also noted at 27 weeks-of-age. After this time point, the percentage of cells in the A fraction remained decreased and the percentage of cells in the C fraction recovered. The reason for the recovery and fraction C is currently unknown. While not statistically significant, there appeared to be a decrease in the percentage of cells within the D fraction at 37 weeks-of-age (Figure 2D). Cells within the F fraction progressively increased as the mice aged and reached statistical significance at 37 weeks-of-age.
Overall, we have shown that alterations in the development and differentiation of B cells within the bone marrow progressively occur during mid- to late-stage disease in NZB/W mice. These data together with our previous results suggest that B cells are transitioning through later stages of development faster and allowing autoreactive B cells to bypass regulatory checkpoints with subsequent release into the circulation [10].
Initiation of HDAC6 inhibition during early disease increases the percentage of bone marrow cells within Hardy fractions B, D, and E
In our previous studies, we have shown correction of aberrant development and differentiation of B cells in the bone marrow of NZB/W mice after selective HDAC6 inhibition [10]. In our present studies, we investigated when initiation of selective HDAC6 inhibition would be most effective in correcting B cell development in the bone marrow. We first evaluated selective HDAC6 inhibition in early disease by treating NZB/W mice with increasing doses of ACY-1083 (0.3 mg/kg, 1 mg/kg, and 3 mg/kg) at 21 weeks-of-age (Figure 3). Another group of mice were treated with dexamethasone to serve as a positive treatment control group. After 13 weeks of treatment, there was a significant dose-dependent decrease in lupus nephritis and spleen size was decreased in mice treated with ACY-1083 compared to controls (unpublished data).
Figure 3: Development and differentiation of B cells in the bone marrow of NZB/W F1 female mice after HDAC6 inhibition initiated early in disease. Mice were treated with ACY-1083 (0.3 mg/kg, 1 mg/kg or 3 mg/kg), or 2 mg/kg dexamethasone (DEX) for 13 weeks, then progression of bone marrow cells through Hardy fractions was evaluated by flow cytometry. (A) There is a dose-dependent increase in the percentage of B220+CD43+ (Pre B) cells after treatment with ACY-1083. Treatment with DEX significantly decreased the percentages of B220+CD43- (Pro B) and B220+CD43+ (Pre B) cells in the bone marrow. (B) Treatment with 1 mg/kg or 3 mg/kg ACY-1083 increased percentages of Pro B cells in the B (CD24+BP-) fraction. Treatment with DEX significantly decreased percentages in the B fraction. (C). There is an increase in the percentages of Pre B cells in the D (IgM-B220Lo), E (IgM+B220Lo), and F (IgM+B220Hi) fractions after treatment with ACY-1083, which reaches statistical significance in the group treated with 3 mg/kg for the E fraction. Treatment with DEX significantly decreased the percentages of cells in fractions D, E, and F. (n ≥ 8; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
To evaluate B cell development and differentiation bone marrow cells were collected then sorted into Hardy fractions as previously described [10]. Treatment with ACY-1083 at all investigated doses had no effect on the percentage of Pro B (B220+CD43+) cells in the bone marrow (Figure 3A). A dosedependent increase in the percentage of Pre B (B220+CD43-) cells in the bone marrow was noted after ACY-1083 treatment, which reached statistical significance in the 3 mg/kg treatment group (p>0.001). Dexamethasone treatment (DEX, 2 mg/kg) significantly reduced the percentages of Pro and Pre B cells in the bone marrow (p<0.0001).
Treatment with 0.3 mg/kg ACY-1083 and 1 mg/kg ACY-1083 increased the amount of Pro B cells in the B fraction (p>0.05) compared to vehicle (HPMC) treated mice (Figure 3B). In contrast, DEX treatment significantly reduced the amount of Pro B cells in the B fraction (p>0.001). Percentages of cells in the A, C, and C’ fractions were not significantly altered by treatment with all doses of ACY-1083 (0.3, 1, and 3 mg/kg) and DEX.
There was a dose-dependent increase in the percentage of cells within the E fraction, which reaches statistical significance in mice treated with 3 mg/kg ACY-1083 compared to vehicle controls (Figure 3C). There was also an increase in the percentages of cells in the D and F fractions after ACY-1083 treatment, although not statistically significant. Treatment with DEX significantly depleted cells in fractions D, E, and F (p<0.0001).
Oral administration of a selective HDAC6 inhibitor decreases spleen size and lupus nephritis when initiated during early disease
A similar selective HDAC6 inhibitor, ACY-738 was formulated in rodent chow and utilized in a pilot study where mice were fed ad libitum with either the ACY-738-formulated chow or standard rodent chow for 18 weeks starting at 16 weeks-of-age (Figure 4). At the same time, another group of NZB/W mice were treated with DEX to serve as a positive treatment control group. The ACY-738 rodent chow was formulated to deliver 100 mg/kg/day to achieve an estimated plasma concentration of 100 nM and generously provided by Acetylon Pharmaceuticals. The mean plasma concentration of ACY-738 at different time intervals was 57.3 ng/mL as determined by mass spectrometry (LC/MS) by Agilux (Worcester, MA, USA).
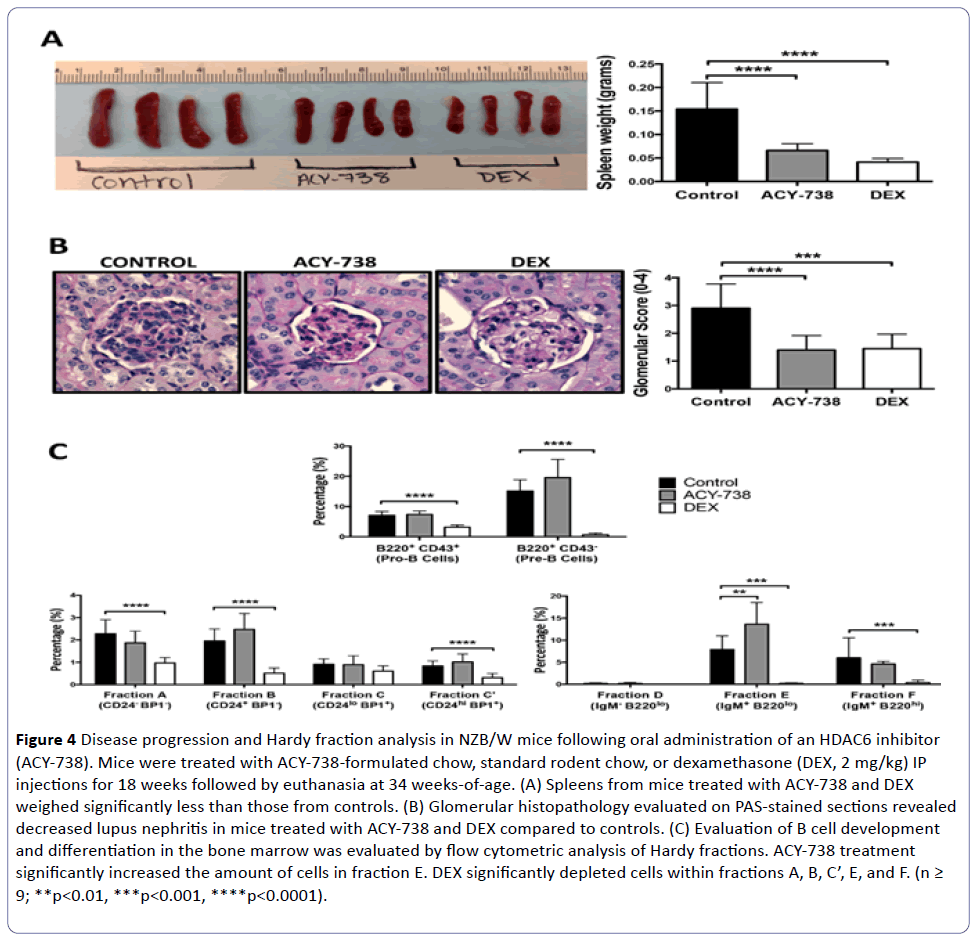
Figure 4: Disease progression and Hardy fraction analysis in NZB/W mice following oral administration of an HDAC6 inhibitor (ACY-738). Mice were treated with ACY-738-formulated chow, standard rodent chow, or dexamethasone (DEX, 2 mg/kg) IP injections for 18 weeks followed by euthanasia at 34 weeks-of-age. (A) Spleens from mice treated with ACY-738 and DEX weighed significantly less than those from controls. (B) Glomerular histopathology evaluated on PAS-stained sections revealed decreased lupus nephritis in mice treated with ACY-738 and DEX compared to controls. (C) Evaluation of B cell development and differentiation in the bone marrow was evaluated by flow cytometric analysis of Hardy fractions. ACY-738 treatment significantly increased the amount of cells in fraction E. DEX significantly depleted cells within fractions A, B, C’, E, and F. (n ≥ 9; **p<0.01, ***p<0.001, ****p<0.0001).
After euthanasia, spleens and kidneys were removed to evaluate disease progression. In mice fed ACY-738, there was a significant decrease in spleen weight (p<0.0001) and lupus nephritis (p<0.0001) when compared to mice fed standard chow, which was similar to the decreased parameters in mice treated with DEX (Figure 4A and 4B).
B cell development and differentiation was also evaluated in these mice by flow cytometric analysis of Hardy fractions in the bone marrow. Following treatment with ACY-738, there was no change in the percentage of Pro B cells but there was an increase in the percentage of Pre B cells in the bone marrow, although not statistically significant (Figure 4C). Furthermore, there was a significant increase in the percentage of cells within fraction E (p<0.01), and an increase in the percentage of cells in fraction B (not statistically significant). These shifts in percentages of B cells within Hardy fractions were similar to those found after NZB/W mice were treated with ACY-1083 (Figure 3).
High-dose HDAC6i initiated during late-stage disease did not alter proportions of bone marrow B cells in developmental Hardy fractions
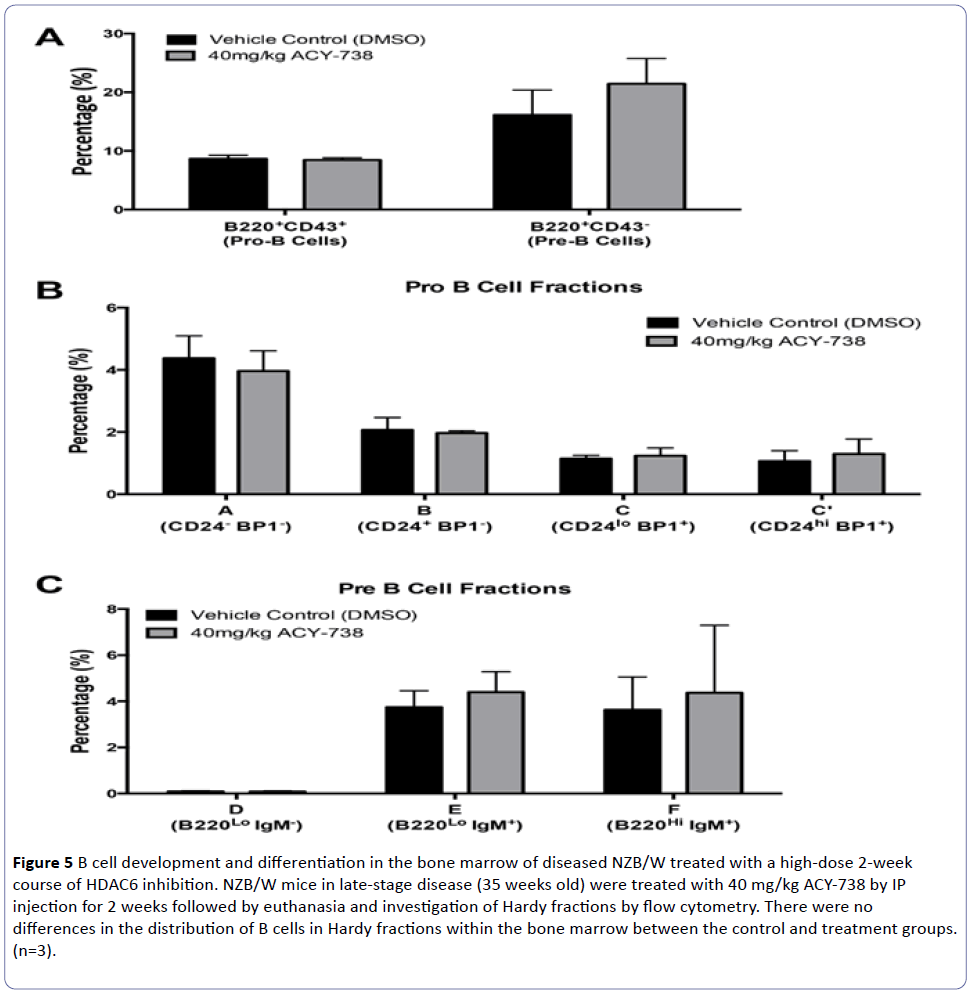
To determine if HDAC6 inhibition would alter bone marrow development in late-stage disease in NZB/W mice, we treated 35-week-old NZB/W mice with 40 mg/kg of the HDAC6 inhibitor ACY-738 (Figure 5). The mice were treated for two weeks after which they were euthanized and bone marrow cells collected and sorted into Hardy fractions. There were no significant differences between mice treated with the HDAC6 inhibitor and those treated with vehicle (DMSO) alone in regards to percentages of bone marrow cells within any of the Hardy fractions (Figure 5). Of particular interest, the percentage of cells within fraction D (Figure 5C) was extremely low in mice treated with either the HDAC6 inhibitor (mean=0.087%) or vehicle (mean=0.091%).
Figure 5: B cell development and differentiation in the bone marrow of diseased NZB/W treated with a high-dose 2-week course of HDAC6 inhibition. NZB/W mice in late-stage disease (35 weeks old) were treated with 40 mg/kg ACY-738 by IP injection for 2 weeks followed by euthanasia and investigation of Hardy fractions by flow cytometry. There were no differences in the distribution of B cells in Hardy fractions within the bone marrow between the control and treatment groups. (n=3).
In addition to no alterations in proportions of bone marrow cells in Hardy fractions after HDAC6 inhibition, there were also no changes in clinical disease parameters or lupus nephritis in these mice (data not shown). Our results showed that treatment initiated during late-stage disease (35 weeks-of-age) was not able to decrease lupus nephritis, which had progressed to irreversible stages of damage.
Genes related to B cell development and differentiation are differentially expressed in the bone marrow after HDAC6i in NZB/W mice
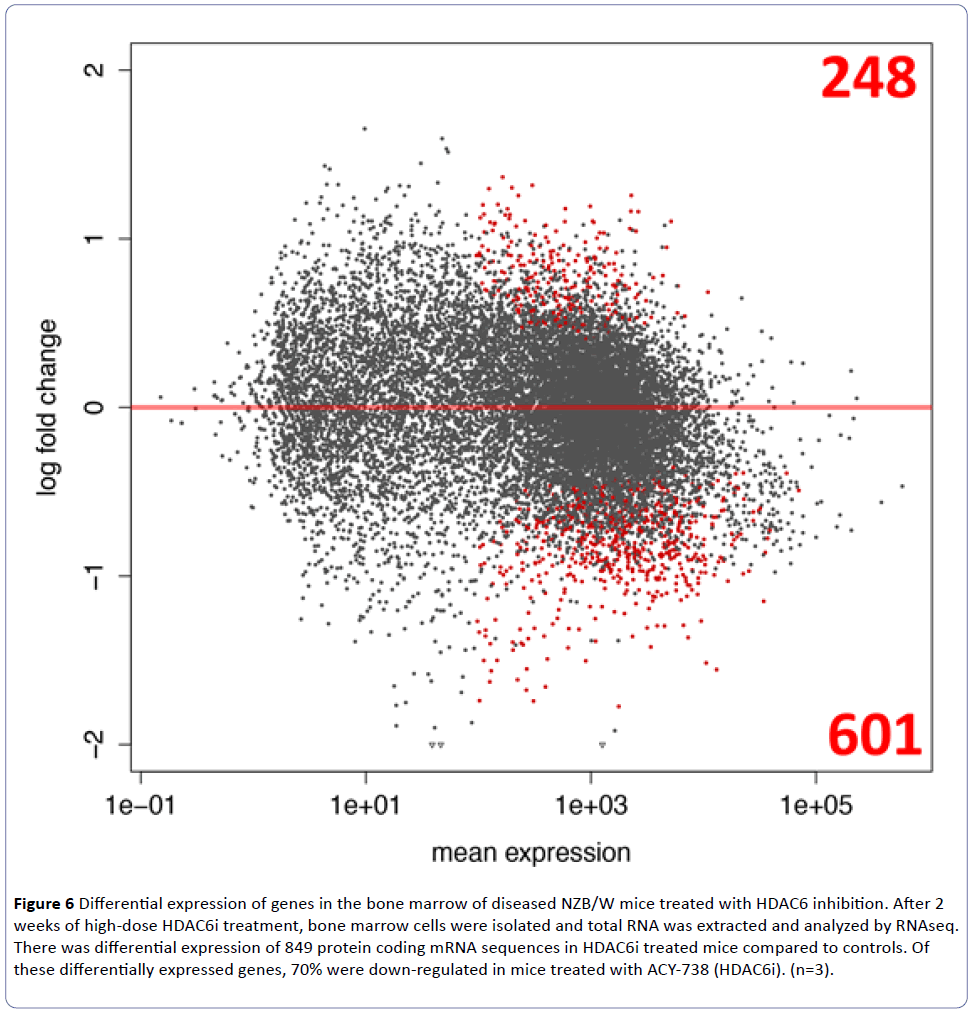
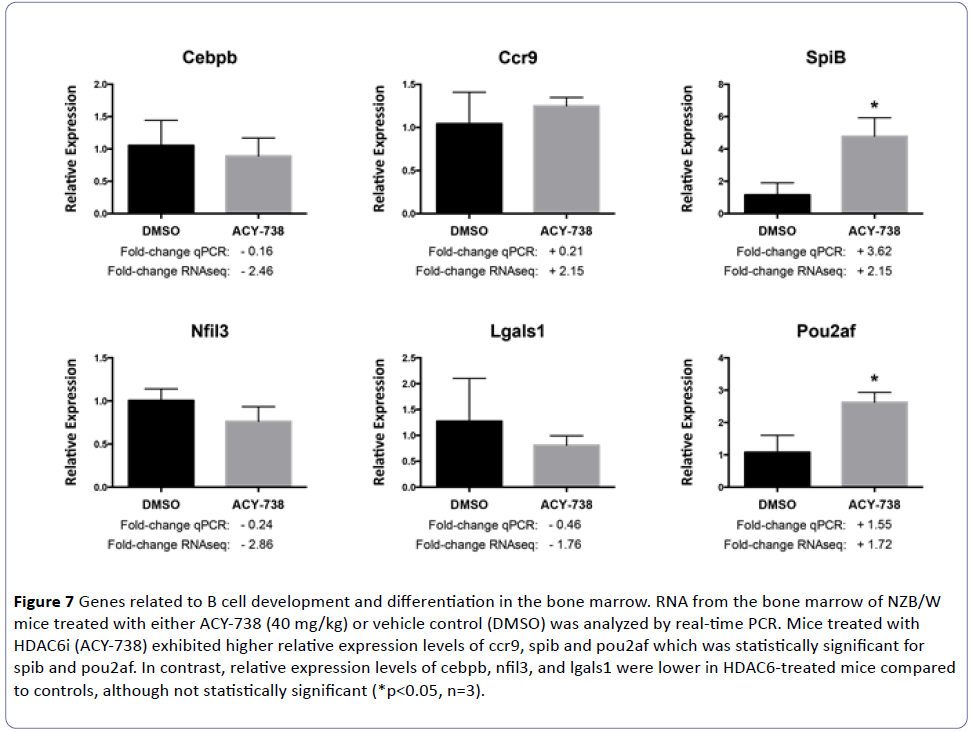
Substrates of HDAC6 related to B cell development and differentiation in the bone marrow are unknown. To identify potential targets of HDAC6 regulation, total RNA was extracted from bone marrow cells of the NZB/W mice treated with a high-dose (40 mg/kg) of ACY-738 for two weeks. The RNA was analyzed by RNAseq to unveil genes involved in B cell development and differentiation that may be regulated by HDAC6. A total of 849 protein-coding mRNA sequences were differentially expressed in mice treated with ACY-738 compared to those treated with vehicle alone (Figure 6). Of these mRNA sequences, 601 were significantly down-regulated and 248 were significantly up-regulated (Supplementary Table 1). Of the genes differentially regulated, six transcripts involved in the regulation of B cell development and differentiation in the bone marrow were selected for further investigation: cebpb, ccr9, spib, nfil3, lgals1, and pou2af1 (Table 1 and Figure 7).
Figure 6: Differential expression of genes in the bone marrow of diseased NZB/W mice treated with HDAC6 inhibition. After 2 weeks of high-dose HDAC6i treatment, bone marrow cells were isolated and total RNA was extracted and analyzed by RNAseq. There was differential expression of 849 protein coding mRNA sequences in HDAC6i treated mice compared to controls. Of these differentially expressed genes, 70% were down-regulated in mice treated with ACY-738 (HDAC6i). (n=3).
Figure 7: Genes related to B cell development and differentiation in the bone marrow. RNA from the bone marrow of NZB/W mice treated with either ACY-738 (40 mg/kg) or vehicle control (DMSO) was analyzed by real-time PCR. Mice treated with HDAC6i (ACY-738) exhibited higher relative expression levels of ccr9, spib and pou2af which was statistically significant for spib and pou2af. In contrast, relative expression levels of cebpb, nfil3, and lgals1 were lower in HDAC6-treated mice compared to controls, although not statistically significant (*p<0.05, n=3).
| Gene | Mean Expression | Fold-Change | p-value | Adjusted p-value |
|---|---|---|---|---|
| Ccr9 Chemokine (C-C motif) receptor 9 |
259.69655 | 2.1538 | 0.00075 | 0.03178 |
| Spib Spi-B transcription factor (spi-1/PU.1) |
5150.14736 | 2.14819 | 0.00467 | 0.07917 |
| Pou2af1 Pou domain, class 2, associating factor (BOB.1/0) |
4348.51165 | 1.71947 | 0.00062 | 0.02801 |
| Nfil3 Nuclear factor, interleukin 3, regulated |
1439.04321 | -1.76353 | 0.00017 | 0.01308 |
| Cebpb CCAAT/enhancer binding protein (C/EBP), beta |
4501.85776 | -2.45591 | 0.00074 | 0.03143 |
| Lgals1 Lectin, galactose binding, soluble 1 |
10559.35381 | -2.85919 | 2.93E-15 | 7.64E-12 |
Table 1: Differentially expressed genes in the bone marrow related to B cell development and differentiation after HDAC6 inhibition based on RNAseq analysis.
CCAAT/enhancer-binding protein β (C/EBPβ) is a transcription factor that is crucial for the differentiation of a variety of cell types and also plays a role in hematopoiesis [19]. C/EBPβ knockout mice exhibited a significant reduction in the number of B220+ cells in the bone marrow [20,21]. Specifically, this reduction was attributed to lower percentages of cells in Hardy fractions A, B, C, and C’ [21]. Ultimately, the reduction in B220+CD43+ pro B cells in the bone marrow of C/ EBPβ-deficient mice was due to the impaired ability of bone marrow mesenchymal stromal cells to support differentiation of hematopoietic stem cells into precursor B cells [21,22]. After selective HDAC6 inhibition, there was a 2.46-fold decrease in cebpb expression according to RNAseq analysis. According to the literature, this should lead to decreased percentages of cells within Fractions A-C’. We saw decreased cell percentages in fractions A, C, and C’ after HDAC6 inhibition, however they were not statistically significant.
CC chemokine receptor 9 (CCR9) is a receptor for CCL25 (thymus-expressed chemokine, TECK). In mice, bone marrow cells phenotypically residing in Hardy Fraction A (pre-pro B cells) migrate in response to TECK and can further generate pro B colony forming units in the presence of IL-7 and Flt-3. Beyond the pre-pro stage, B lineage cells in the bone marrow and periphery lack a migratory response to TECK. It is postulated that responsiveness to TECK may direct pre-pro B cells into specialized niches that are supportive to their developmental stage. Additionally, TECK may play a role in total B cell output by regulating the total number of B lineage cells allowed to develop [22]. In support of this theory, when CCR9 is knocked out in mice, there is a 3-fold decrease in the number of pre-pro B cells in the bone marrow compared to wild type. However, these CCR9-/- mice exhibit a normal complement of mature B cells, suggesting a homeostatic adjustment occurring within downstream developmental stages [23]. When treated with the selective HDAC6 inhibitor ACY-738, there is a 2.15-fold increase in ccr9. Based on the literature, we might expect an increase in the percentage of cells in early B cell fractions. While amounts of cells in Fraction A were relatively unchanged, we saw an increase in the percentage of cells within Fraction B after selective HDAC6 inhibition. Additionally, as the mice age and develop disease, the percentage of cells within Fractions A and B decrease. Whether or not ccr9 is involved in altering B cell development and differentiation in the bone marrow and subsequently contributes to SLE disease is unknown.
Of the 3 genes involved in early B cell differentiation and development in the bone marrow (cebpb, ccr9, and spib), the most differentially regulated gene after HDAC6 inhibition was spib. Spi-B is an Ets-family transcription factor that shares a high degree of identity with PU.1 including an indistinguishable DNA binding specificity [24,25]. Spi-B can substitute for PU.1 and promote differentiation of hematopoietic progenitor cells into pro B cells, however it is not required [26]. Furthermore, mice deficient in Spi-B do not exhibit any abnormalities in lymphoid development [27]. After HDAC6 inhibition in NZB/W mice, spib was upregulated by 2.15-fold according to RNAseq analysis and 3.62-fold by qPCR. The consequences of increased Spi-B in the bone marrow and whether or not there are altered levels of Spi-B as SLE progresses are both unknown.
Nuclear factor, interleukin-3 regulated (NFIL3) protein, also known as E4BP4, is a basic leucine zipper transcription factor that has roles in diverse hematopoietic lineages including NK cells, T cells, and dendritic cells [28]. In regards to B cells, E4BP4 has been implicated in controlling IgE production and class switching in response to IL-4 [29,30]. More interestingly, NFIL3/E4BP4 is also involved in a group of IL-3-dependent signaling pathways that regulate the survival of murine pro B lymphocytes [31]. More specifically, IL-3-dependent suppression of apoptosis may be accomplished through induction of NFIL3 [31]. If NFIL3 is elevated in SLE, it may be contributing to decreased apoptosis of autoreactive B cells at regulatory checkpoints in the bone marrow. If this were the case, it would be ideal to decrease NFIL3 to allow apoptosis to resume. After HDAC6 inhibition, RNAseq analysis reveals a 2.86-fold decrease in nfil3 expression.
Galectin-1 (GAL1), encoded by the lgals1 gene, is expressed in the bone marrow by osteoblasts and reticular cells and creates a stromal cell niche for pre-BII cells (Fraction D) [32]. Within this niche, interactions between GAL1 and pre B cells create an immune developmental synapse promoting pre-BCR clustering, signaling, and activation [33,34]. Inhibition of pre- BCR/GAL1/integrin interactions in normal pre-BII cells impairs in vitro B cell differentiation, and pre-BII cells from GAL1- deficient mice also exhibit decreased differentiation and proliferation [35]. Following HDAC6 inhibition, RNAseq analysis showed decreased lgals1 in the bone marrow, which should lead to decreased differentiation and proliferation. Instead, we saw an increased percentage of cells in Fraction D. As NZB/W mice age there is a decrease in the percentage of cells in fraction D raising the possibility that decreased lgals1 may play a role in later stages of SLE disease. However, GAL1-deficient mice do not exhibit a B cell phenotype [35].
Pou domain, class 2, associating factor (Pou2af1), also known as BOB.1/OBF.1, is a transcriptional coactivator that works in concert with Oct1 and Oct2 in developing B cells. In BOB.1/OBF.1-deficient mice there is a reduction in transitional immature B cells (Fractions E and F) and an increase in B cell apoptosis in the bone marrow suggesting an important role for BOB.1/OBF.1 in early B cell differentiation and survival [36]. After HDAC6 inhibition, RNAseq analysis showed a 1.72-fold increase in pou2af1 in the bone marrow and the percentage of cells in fraction E increased. Furthermore, qPCR showed a 1.55-fold increase in pou2af1 in the bone marrow after HDAC6 inhibition, implying HDAC6 plays a role in regulating pou2af1.
Total RNA from the bone marrow was also reverse transcribed into cDNA, then analyzed by real-time PCR to quantitate relative expression levels of cebpb, ccr9, spib, nfil3, lgals1, and pou2af1. The relative expression levels of pou2af and spiB were significantly higher in mice treated with ACY-738 compared to vehicle controls (p<0.05) (Figure 7). There was also higher ccr9 expression in HDAC6i-treated mice, however it was not statistically significant. In contrast, the expression levels of cebpb, lgals1, and nfil3 were lower in HDAC6i-treated mice compared to controls, albeit not statistically significant.
Summary and Conclusions
Autoreactive B cells are a major contributor to disease in SLE. The first regulatory control mechanism where autoreactive B cells should be removed or rendered anergic is within the bone marrow [1]. Previous studies have shown aberrant development and differentiation of B cells in the bone marrow of NZB/W mice and correction after selective HDAC6 inhibition [10]. We have shown that alterations in the development and differentiation of B cells within the bone marrow progressively occur during mid- to late-stage disease in NZB/W mice. More specifically, B cells within the bone marrow appear to transition more quickly into the later stages of development (Fraction F) and bypassing important regulatory checkpoints to maintain central tolerance. Treatment with a selective HDAC6 inhibitor is able to correct these changes when initiated during the early stages of disease by apparently slowing progression through developmental stages. Therefore, the cells are able progress through regulatory checkpoints more efficiently. After deep sequencing analysis, we investigated the differential expression of 6 genes related to B cell development and differentiation and whether they could be regulated by HDAC6. HDAC6 appears to have the most impact on expression of spiB, an early regulator of B cell development, and pou2af1, a regulator during later stages of B cell development. Differential expression of these 6 genes and their contribution to the progression of SLE disease is uncertain at this point and warrants further investigation.
Acknowledgements
We would like to acknowledge Melissa Makris for her expertise and assistance in operation of the flow cytometer and subsequent analysis. We also would like to thank the teaching and research animal care support services (TRACCS) staff at Virginia-Maryland College of Veterinary Medicine for their attention to our animals. We also appreciate the support from Acetylon Pharmaceuticals and their generous donation of the HDAC6 inhibitors utilized in these studies.
Conflict of Interest
The authors do not have any conflicts of interest to disclose.
Supplementary Information
Supplementary information is available online.
Funding
This study is funded by Acetylon Pharmaceuticals, Inc. (www.acetylon.com) and the National Institutes of Health (R15AR062883)
References
- Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, et al. (2005) Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 201: 703-711.
- Morton JI, Siegel BV, Moore RD (1975) Transplantation of autoimmune potential II. Glomerulonephritis in lethally irradiated DBA/2 recipients of NZB bone marrow cells. Transplantation 19: 464-469.
- Meffre E, Wardemann H (2008) B-cell tolerance checkpoints in health and autoimmunity.Curr Opin Immunol 20: 632-638.
- Kondo M, Weissman IL, Akashi K (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91: 661-672.
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K (2012) Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. 1991. J Immunol 189: 3271-3283.
- Osmond DG, Rolink A, Melchers F (1998) Murine B lymphopoiesis: towards a unified model. Immunol Today 19: 65-68.
- Opstelten D, Osmond DG (1983) Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu-chain-bearing cells in normal mice. J Immunol 131: 2635-2640.
- Park YH, Osmond DG (1987) Phenotype and proliferation of early B lymphocyte precursor cells in mouse bone marrow. J Exp Med 165: 444-458.
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, et al. (2003) Predominant autoantibody production by early human B cell precursors. Science (New York, NY) 301: 1374-1377.
- Regna NL, Vieson MD, Luo XM, Chafin CB, Puthiyaveetil AG, et al. (2015) Specific HDAC6 inhibition by ACY-738 reduces SLE pathogenesis in NZB/W mice. Clin Immunol(Orlando, Fla) 162: 58-73.
- Vieson MD, Reilly CM (2015) Regulation of non-histone proteins by HDAC6 in systemic lupus erythematosus. Curr Trends Immunol 16: 93-103.
- Regna NL, Vieson MD, Gojmerac AM, Luo XM, Caudell DL, et al. (2015) HDAC expression and activity is upregulated in diseased lupus-prone mice. Int Immunopharmacol.
- Aronesty E (2011) Command-line tools for processing biological sequencing data.
- Kong Y (2011) Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98: 152-153.
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biol 14: R36.
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. NatMethods 9: 357-369.
- Anders S, Pyl PT, Huber W (2015) HTSeq: A Python framework to work with high-throughput sequencing data. Bioinformatics (Oxford, England) 31: 166-169.
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550.
- Tsukada J, Yoshida Y, Kominato Y, Auron PE (2011) The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54: 6-19.
- Chen X, Liu W, Ambrosino C, Ruocco MR, Poli V, et al. (1997) Impaired generation of bone marrow B lymphocytes in mice deficient in C/EBPbeta. Blood 90:156-164.
- Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, et al. (2014) CCAAT/enhancer-binding protein beta expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem cells (Dayton, Ohio) 32: 730-740.
- Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, et al. (2000) Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med 191: 1303-1318.
- Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, et al. (2001) Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood 98: 2626-2632.
- Ray D, Bosselut R, Ghysdael J, Mattei MG, Tavitian A, et al. (1992) Characterization of Spi-B, a transcription factor related to the putative oncoprotein Spi-1/PU.1. Mol Cell Biol 12: 4297-4304.
- Ray-Gallet D, Mao C, Tavitian A, Moreau-Gachelin F (1995) DNA binding specificities of Spi-1/PU.1 and Spi-B transcription factors and identification of a Spi-1/Spi-B binding site in the c-fes/c-fps promoter. Oncogene 11: 303-313.
- DeKoter RP, Lee HJ, Singh H (2002) PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity16: 297-309.
- Su GH, Chen HM, Muthusamy N, Garrett-Sinha LA, Baunoch D, et al. (1997) Defective B cell receptor-mediated responses in mice lacking the Ets protein, Spi-B. The EMBO journal 16: 7118-29.
- Male V, Nisoli I, Gascoyne DM, Brady HJ (2012) E4BP4: an unexpected player in the immune response. Trends Immunol 33: 98-102.
- Kashiwada M, Levy DM, McKeag L, Murray K, Schroder AJ, et al. (2010) IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci USA 107: 821-826.
- Rothman PB (2010) The transcriptional regulator NFIL3 controls IgE production. Trans Am Clin Climatol Assoc 121: 156-71.
- Kuribara R, Kinoshita T, Miyajima A, Shinjyo T, Yoshihara T, et al. (1999) Two distinct interleukin-3-mediated signal pathways, Ras-NFIL3 (E4BP4) and Bcl-xL, regulate the survival of murine pro-B lymphocytes. Mol Cell Biol 19: 2754-2762.
- Mourcin F, Breton C, Tellier J, Narang P, Chasson L, et al. (2011) Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 117: 6552-6561.
- Gauthier L, Rossi B, Roux F, Termine E, Schiff C (2002) Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA 99:13014-13019.
- Rossi B, Espeli M, Schiff C, Gauthier L (2006) Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol 177: 796-803.
- Espeli M, Mancini SJ, Breton C, Poirier F, Schiff C (2009) Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood 113: 5878-5886.
- Hess J, Nielsen PJ, Fischer KD, Bujard H, Wirth T (2001) The B lymphocyte-specific coactivator BOB.1/OBF.1 is required at multiple stages of B cell development. Mol Cell Biol 21: 1531-1539.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences