The Discovery of Two Novel Biomarkers in a High-Fat Diet C56bl6 Obese Mouse Model for Non-Adipose Tissue: A Comprehensive LCMS Study at Hind Limb, Heart, Carcass Muscle, Liver, Brain, Blood Plasma and Food Composition Following a Lipidomics LCMS-Based Approach
van Ginneken V, Verheij, de Vries and van der Greef J
DOI10.21767/2573-5365.100022
van Ginneken V1*, Verheij E2, de Vries E1 and van der Greef J2,3
1Blue Green Technologies, Runderweg 6, 8219 PK, Lelystad, Netherlands
2TNO Pharma, AJ Zeist, The Netherlands
3Sino-Dutch Center for Preventive and Personalized Medicine, Leiden University, RA Leiden, Netherlands
- *Corresponding Author:
- van Ginneken V
Blue Green Technologies, Runderweg 6
8219 PK, Lelystad, Netherlands
Tel: 0031638071180
E-mail: vvanginneken@hotmail.com
Received Date: October 28, 2016; Accepted Date: November 21, 2016; Published Date: November 24, 2016
Citation: Ginneken V, Verheij E, de Vries E, et al. The discovery of two novel biomarkers in a high-fat diet C56bl6 obese model for non-adipose tissue: A comprehensive lcms study at hind limb, heart, carcass muscle, liver, brain, blood plasma and food composition following a lipidomics lcms-based approach. Cell Mol Med 2016, 2:3.
Abstract
Aim/Objective: This study was designed to find via a highfat (HF) diet induced insulin resistant (IR) and/or type-2 diabetes (T2DM) C57Bl/6 mouse model potential novel biomarkers. Major aiming is to find following this lipidomics based approach novel safe biomarkers applicable for humans with IR/T2DM that can be used in the assessment of diagnosis, intensive treatment, clinical use and new drug development. In addition, the biomarker has to be found in blood-plasma simultaneously while is not a component of the HF-diet.
Methods: Reversed phase liquid chromatography coupled to mass spectrometry (LC-MS) were used to quantify and qualify the rearrangement and repartitioning of fat stores in the heart-, hind limb-, carcass-muscle, liver, brain, and blood plasma of this mice model following a systems biology lipidomics based approach.
Results: Two potential biomarkers were found for this HFdiet mouse model. The first biomarker was a 20:3 cholesteryl-ester (20:3-ChE) which significantly increased (P ≤ 0.016) in the fatty heart with 1317% while it rose very significantly (P ≤ 0.00001) in blood plasma with 1013% in the HF diet group in comparison to the control-group. (Co). The second biomarker was a 36:1 phosphatidylcholine (36:1-PC), which rose significantly (P ≤ 0.025) mainly in heart muscle with 400% while concentrations increased significantly strongly (P ≤ 0.002) in blood plasma with 1493% in the high-fat diet vs. Co. As an earlier defined prerequisite, both compounds were not found in the food.
Conclusion: The 20:3-ChE biomarker (dihomo-γ-linolenic; 20:3 n-6) has been classified as a potential type 2 diabetes biomarker (T2DM) biomarker in a human cohort of the uppsala longitudinal study of adult men (ULSAM). In addition, we give a biochemical explanation for the 36:1- PC as hypoxic biomarker for cardiovascular diseases (CVD) diagnosis and therapy. Both biomarkers are interesting candidates for further validation in human cohorts.
Keywords
Systems biology; Lipidomics; Insulin resistance; Type-2 diabetes; Cardiovascular diseases (CVD); High-fat diet mouse model; Biomarker; Phosphatidylcholine; Cholesteryl-esters
Introduction
Over the past century, traditionally medical care has centered on standards of care based on epidemiological studies of large cohorts. However, large cohort studies do not take into account the genetic variability of individuals within a population. E.g. at the level of genomics, according the national human genome research (NHGRI) about 99.9% of the DNA sequence is identical in all people, but the 0.1% difference is critical because it represents the genetic variations in the susceptibility of a person for getting a disease, the severity of the disease, and what is the impact of the medical/therapeutic treatment [1]. In “traditional” medicine a “Personalized Medical Approach” has been limited to the consideration of a patient’s family history, social circumstances, environment and behaviors in tailoring individual care [2]. The traditional reductionistic approach has led using animal rodent-models which have uncovered many potential candidate for diabetes susceptibility genes [3,4]. In general it as assumed type 2 diabetes T2DM is a polygenic disorder and is the result of interaction between environmental factors and a strong hereditary component [5].
However, an “one single gene” approach might only in a few percentages of the T2DM be the cause. Therefore, a systemsbiology approach is clearly warranted. At the cellular/organ/ tissue level of the organism one of the challenges of measuring metabolite levels within the research area metabolomics approach in human metabolism is determining the significance of metabolomic changes. Metabolomics is a discipline dedicated to the systematic study of small molecules (i.e. metabolites) in cells, tissues, and different bio-fluids. Metabolite levels can be regarded as amplified responses of biological systems to genetic or environmental changes [6].
The challenge of a metabolomics approach is not only the discovery of changes in metabolite profile but it is figuring out what these changes mean which ultimately can lead to a biomarker for a pathogenesis. By a systems-biology approach [7] and the search for new biomarkers applicable for insulin resistance (IR) and type 2 diabetes (T2DM) in a “Personalized Medical Approach”, this would provide a scientific baseline for clinical trials and new medicines to treat IR and T2DM.
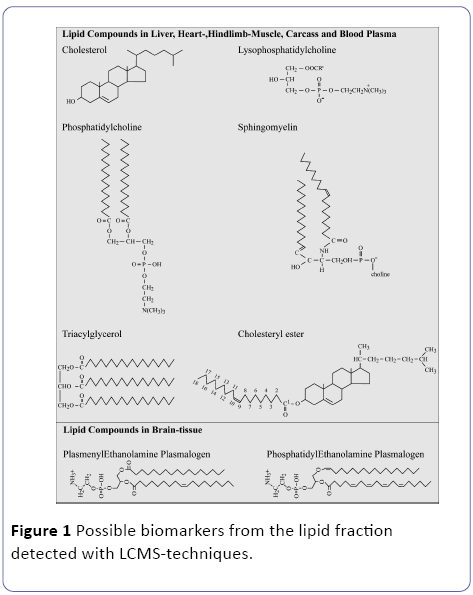
A particular area of interest of metabolomics based systemsbiology, will be identification of novel safety lipidomics biomarkers (Figure 1), that can be used in the assessment of new intensive treatments both during their discovery phase and in clinical use to study e.g. metabolism-related diseases like T2DM in combining the diagnostics with the patient therapy [8]. A biomarker is defined as a substance used as an indicator of a biological state. It is characteristic that it is objectively measured and evaluated as an indicator of normal biological processes, nutritional intervention, pathogenic processes, or pharmacological responses to a therapeutic intervention: exercise, nutritional and pharmacological intervention [6]. Obesity, excessive caloric input of unhealthy food, ageing and physical inactivity the disease prevention therapies are generally well defined for the present T2DM patients.
The search for reliable in clinical human studies applicable biomarkers for obesity/T2DM would provide a scientific baseline in personalized medicine for clinical patient directed trials of anti-obesity/T2DM medicines and the effects of the recently development of "functional foods" and give an estimation of the effect of an exercise protocol exposure during the treatment of these metabolic diseases [9,10]. A demand for a biomarker of the lipid fraction is that homeostatic conditions are needed for the biomarker to reflect accurately long-term intake and not to be biased by lipolysis [11]. Fatty acids can be measured as free fatty acids in serum, components of circulating triacylglycerols, components of erythrocyte membranes, phospholipids or cholesteryl esters, or adipose tissue from various sites. In this respect, molecular mediators in blood plasma are important to study because they can serve as biomarkers to trace people vulnerable and susceptible for metabolic diseases like Obesity and/or IR/T2DM [12].
Recent technological advances in metabolomics [8,13,14] and lipidomics [15] can potentially make a real contribution in increasing efficacy of drug development pipelines[6]. In Figure 1 are some common lipid compounds given for non-adipose tissue which are suitable as biomarker following a systems biology lipidomics approach. Our initial hypothesis is that in excessive white adipose tissue (WAT) hypoxia occurs due to a low vascularization as depicted in Figure 2 and described by[16].
We hypothesize that in these non-adipose tissues due to the accumulation of WAT hypoxic conditions will occur with all kind of deleterious effects on the tissues related to hypoxia [17]. In addition, in this manuscript we will give the final results of a high-Fat diet induced obese mouse model in relation to the found biomarkers in non-adipose tissue. We hope this manuscript will contribute to elucidate the major conversions of lipid compounds and will serve as a valuable resource to a broad range of researchers and students involved in various aspects of mammalian lipid science [18].
Material and Methods
Experimental animals
In order to find biomarkers in the lipid fraction based on a lipidomics approach of different non-adipose tissues and organs (liver, heart-, carcass-, hind limb-muscle, brain including blood-plasma) we administered two different diets (one regular and one high-fat [HF] diet) on a specific mouse model, the mouse strain C57BL/6 and compared the lipid profiles using LCMS techniques. Purebred male wild-type C57bl6 mice (age 8-12 weeks), obtained from Charles river (Maastricht, The Netherlands) were used. Animal experiments were approved by the animal experimentation committee of the Leiden University Medical Centre (The Netherlands). Two feeding regimes were compared: 1. a regular diet (Control) and 2. a high-dietary situation in which mice received a high-fat diet during 40 days and then were sacrificed (Treatment). In total, 13 rodents were used: 6 mice in the Control group and 7 in the Treatment group. Mice were housed in a temperaturecontrolled room (21°C) on a 10 hour dark/14 hour light cycle.
Diet
Mice in the Control group were fed a standard lab chow (SDS.3, special diet services, Witham, UK) containing about 4.3 energy percent fat (Table 1). The fatty diet that was fed to mice in the Treatment group contained 21.4% protein, 36% carbohydrates, 24% fat, 6% fibers and 5.7% water (weightpercentages). Before the experiment started animals of both the control group and the treatment group received unrestricted amounts of food and water. Mice in the Control group fasted for 4 hours before the start of the experiment in order to standardize their metabolic rate.
| Proximate analysis | Standard (SDS.3) | Proximate analysis | Fatty diet (4032.05) |
|---|---|---|---|
| Moisture (%) | 10.00 | Moisture (%) | 5.74 |
| Crude oil (%) | 4.25 | Crude fat (Bovine Lard) (%) | 24.00 |
| Crude protein (%) | 22.39 | Crude protein (%) | 21.44 |
| Crude fiber (%) | 4.21 | Crude fiber (%) | 6.16 |
| Ash | 7.56 | Minerals | 2.25 |
| Nitrogen free extract | 51.20 | Nitrogen free extract | 36.19 |
| ----- | - | Cholesterol | 0.25 |
| TOTAL | 99.61 | Total | 96.03 |
| Energy (measured bomb-calorimetry) (kJ/g dm) | 16.86 | Energy (measured bomb-calorimetry) (kJ/g dm) |
21.46 |
Table 1: Food composition of the mice chow: “normal” for Control group (Special Diet Services, SDS No.3, Witham, UK) and the high-fat ≈ “fatty diet” (Arie Blok, food code 4032.05, Woerden, The Netherlands) based on bovine lard and 0.25% cholesterol.
Tissues
A tissue- or feed- homogenate (∼10% wet weight/vol) in PBS (phosphate-buffered saline) was made by stirring the tissue in a closed tube with small glass beads.
Mass spectrometry (LC-MS)
As described earlier [19-21] fifty μl of the well mixed tissue homogenate was mixed with 1000 μl IPA containing 4 internal standards. In addition, blood plasma samples of 10 μl plasma were extracted with 300 μl of isopropanol (IPA) containing several internal standards (IS: C17:0 lysophosphatidyl choline, di-C12:0 phosphatidylcholine, tri-C17:0 glycerol ester, C17:0 cholesterol ester and heptadecanoic acid (C17:0)). Samples were placed in an ultrasonic bath for 5 minutes.
After mixing and centrifugation (10000 rpm for 3 minutes) the supernatant was transferred to an autosampler vial. Thereaftere10 μl of the sample was injected on the LC-MS Instrument (Thermo Electron, San Jose, USA). A Thermo LTQ is a linear ion-trap LC-MS instrument (Thermo Electron, San Jose, USA). Lipids were separated on a 150 × 3.2 mm i.d. C4 Prosphere column (Alltech, USA) using a methanol gradient in 5 mM ammonium acetate and 0.1% formic acid (mobile phase A: 5% methanol, mobile phase B: 90% methanol).
The flowrate was 0.4 ml/min and the gradient was as follows: 0-2 min-20% B, 2-3 min-20% to 80% B, 3-15 min-80% to 100% B, 15-25 min-hold 100% B, 25-32 min-condition at 20% B. The instrument used was a thermo LTQ equipped with a thermo surveyor HPLC pump. Data were acquired by scanning the instrument from m/z 300 to 1200 at a scan rate of approximately 2 scans/s in positive ion ESI mode.
Definition of a Biomarker for the Several Compounds Measured by LCMS Techniques from the Lipid Fraction
Definition of a Biomarker from the 6 major lipid compounds (Figure 1)
At experimental animal (mouse) level a prerequisite for a biomarker is that it can be distinguished from the Control group fed a control-chow e.g. in comparison to our, for 40 days fed a High Fat diet for our C57BL/6 mouse model resulting in obese IR/T2DM animals e.g. by an elevated concentration in an organ or tissue:
A) So, in terms of product-precursor ratio the metabolic route is obstructed, or the enzyme cannot convert in its metabolic route the precursor to its end product resulting in an accumulation of the precursor: the detected biomarker. This means that the metabolic pathways or enzymes are blocked in this way resulting in an accumulation of the end product=> the biomarker.
B) A second prerequisite is that it may not be diet induced which means that it may not be found in the control-chow or high-fat diet.
C) A third requisite for a biomarker in the field is that organ/ tissue sampling by biopsy is very uncomfortable, not practical and sometimes dangerous for e.g. a liver biopsy or impossible in case of heart muscle. Therefore, the third precondition for a suitable biomarker is that it not only accumulates in the specific organ or tissue but also in the blood-plasma. So, accumulation takes place because of a blockage in a specific pathway or metabolic route [19].
In case of Lipidomics these are the major metabolic pathways of n-3 and n-6 PUFA (Figure 3). An approach to get an impression of enzyme activities (endogenous conversion of desaturases and elongases) in the liver (expressed in: change in hepatic expression) can be estimated from product-toprecursor ratios as given in below according to van (Figure 3) [20-22]. Activity of desaturases and elongases of the cholesteryl esthers (ChE) of the blood plasma fraction can be performed using the product-to-precursor ratios of individual LC-MS measured fatty acids as follows:
• C18:3n6/C18:2n6 ratio=Δ6-desaturase
• C20:3n6/C18:3n6 ratio=elongase
• C20:4n6/C20:3n6 ratio=Δ5-desaturase
• C16:1n7/C16:0 ratio=Δ9-desaturase
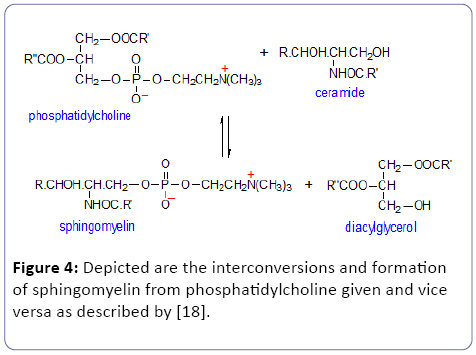
• D) Toxic lipid species: According to our definition for a suitable biomarker toxic compounds are excluded from our bio-medical definition [23-25]. Thus we exclude sphingomyelin because it can be converted to the toxic Ceramide which can cause cellular apoptosis [26].
• E) Sphingomyelin (SPM): is a sphingolipid, located mostly in the outer layer of the plasma membrane. Ceramide is the second messenger in the sphingomyelin signaling pathway (Figure 4). Experimental studies revealed that ceramide might impair insulin action via: 1). maintaining protein kinase B in an inactive dephosphorylated state; 2). a reduction of GLUT4 translocation to plasma membrane; 3). a decrease in insulin-stimulated glucose uptake; 4). Tumor Necrosis Factor (TNF)-α, a well-known mediator of insulin resistance, acts through activation of neutral sphingomyelinase (enzyme which hydrolysis sphingomyelin => ceramide) and induction of ceramide formation (reviewed: Straczkowski et al. 2004).
Calculations and statistics
For all parameter, the mean value of the control mice group was compared to the mean value of the fatty-diet group. Statistics were performed via SPSS using a two-tailed t-test for differences between the control group and the treatment (fatty-diet) group. P ≤ 0.05 was considered as statistically significant. Normality of the data and homogeneity of variances were checked by Kolmogorov-Smirnov and Fmax tests, respectively.
Results
LC-MS-results
An example of a chromatogram of hind limb muscle is displayed in Figure 5. Three groups of chemical compounds can be clearly distinguished in these Figure: A): after 9-11 minutes retention time the lysophosphatidyl cholines (LPC) become visible with at 12 minutes the Internal Standard dilauroyl- phoshatidyl choline (IS); B): after 13-16 minutes the phosphatidylcholines (PC), Sphingomyelins (SPM) and phosphatidylethanolamines (PE) become visible; and C): after 17-19 minutes the triacylglycerols (TG) and cholesterol-esters (ChE).
In this study, we observed clearly tissue specific differences between several non-adipose tissues/organs: liver, heart-, carcass-, hind limb-muscle, brain including blood-plasma in the high fat (HF) diet group in comparison to the control group. As described in the result section (Annex 1), these differences consider mainly cholesteryl esters (ChE), lysophosphatidyl cholines (LPC), phosphatidylcholines (PC) and sphingomyelin (SPM) but due to interconversions and/or toxic compounds we clearly restricted the definition of a bio-medical biomarker. Comparisons of LPC, PC and SPM show no general pattern. Sometimes these compounds were significantly higher or lower in the several non-adipose tissues and organs. Related to hepatic enzymatic activity as defined under M and M we will concentrate on elongase activity because this will result in increased fat =>white adipose tissue (WAT). From Tables 2 and 3 we see that under conditions of starvation elongase activity is significantly not increased but non-significantly decreased until ≈80% in comparison to the co-group but under high-fat diet conditions significantly (P<0.0003***) increased with 304%. So, high-fat diet conditions lead to fat formation according to elongase/desaturase enzymatic acitivity (Figure 3). Because of the interconversion of phosphatidylcholine (PC) towards lysophosphatidyl choline (LPC) in the “Lands Cycle” only the PC lipids are considered as interesting biomarkers. Because sphingomyelin can be converted to the toxic Ceramide (Figure 4) it is not suitable as a biological biomarker [27,28].
| Change in hepatic expression | Control-blood | 24-h starvation | P-value | % change |
|---|---|---|---|---|
| ÃÆâÃâÃâ Ãâââ¬Â 6-desaturase | 0.031 ± 0.003 | 0.036 ± 0.005 | P ≤ 0.034* | 118.0% ↑ |
| Elongase | 1.142 ± 0.272 | 0.914 ± 0.312 | P ≤ 0.170 | 80.0% ≈ |
| ÃÆâÃâÃâ Ãâââ¬Â 5-desaturase | 15.780 ± 1.273 | 32.584 ± 7.321 | P ≤ 0.0008*** | 206.5% ↑ |
| ÃÆâÃâÃâ Ãâââ¬Â 9-desaturase | 2.366 ± 0.342 | 1.388 ± 0.173 | P ≤ 0.0001*** | 58.7 % ↓ |
Table 2: Enzyme activity of desaturases and elongases in blood plasma. With LC-MS techniques the individual fatty acids of the Cholesterol fraction in blood plasma were determined in a Control (N=7) and a 24 hours-starvation (N=7) C57Bl/6 mouse group. Endogenous conversions by desaturases and elongate were estimated from product-to-precursor ratios as given in Material and Methods.
| Change in hepatic expression | 40-days Control-diet | 40-days high-fat diet |
P-value | % change |
|---|---|---|---|---|
| ÃÆâÃâÃâ Ãâââ¬Â 6-desaturase | 0.031 ± 0.001 | 0.092 ± 0.006 | P ≤ 0.00000**** | 296.3 % ↑ |
| Elongase | 1.046 ± 0.092 | 3.181 ± 0.617 | P ≤ 0.0003*** | 304.0 % ↑ |
| ÃÆâÃâÃâ Ãâââ¬Â 5-desaturase | 12.751 ± 0.629 | 3.911 ± 0.768 | P ≤ 0.00000**** | 30.67 % ↓ |
| ÃÆâÃâÃâ Ãâââ¬Â 9-desaturase | 2.661 ± 0.532 | 8.911 ± 1.200 | P ≤ 0.00000**** | 334.52 % ↑ |
Table 3: Enzyme activity of desaturases and elongases in blood plasma. With LC-MS techniques the individual fatty acids of the Cholesterol fraction in blood plasma were determined in a 40 days Control-diet (N=5) and a 40 days high-fat diet (N=6), C57Bl/6 mouse group. Endogenous conversions by desaturases and elongase were estimated from product-to-precursor ratios as given in Material and Methods.
A 36:1 Phosphatidylcholine (PC) 36:1 PC rose significantly in this same mouse model in heart muscle with 403% (P ≤ 0.002) and in hind limb muscle with 196% (P ≤ 0.025) and in blood plasma significantly with +1493% (P ≤ 0.00001). Both components were not found in the food.
A number of cholesteryl esters increased significantly in the heart of the animals on a high-fat diet (Figure 6). Most important observations of this study was that in the high-fat diet induced IR mouse model a 20:3 cholesteryl-ester (20:3- ChE) rose significantly in heart muscle with +1317% (P≤ in the high-fat diet exposed group (in comparison with the Control group) and in blood plasma with +1013% (P ≤ 0.000001) (Figure 7) [29,30].
TGs are not interesting biomarkers because they are determined in great extent by the food.
Discussion
Metabolomics provides a direct functional read-out of the physiological status of an organism and is in principle ideally suited to describe someone’s health status [31]. A demand for a biomarker of the lipid fraction is that homeostatic conditions are needed for the biomarker to reflect accurately long-term intake and not to be biased by lipolysis [11]. Fatty acids can be measured as free fatty acids in serum, components of circulating triacylglycerols, components of erythrocyte membranes, phospholipids or cholesterol esters, or adipose tissue from various sites. In this respect molecular mediators in blood plasma [12] are important to study because they can serve as biomarkers to trace people vulnerable and susceptible for metabolic diseases like Obesity and/or IR/T2DM. Recent technological advances in Metabolomics [13,14,25] and Lipidomics [15] can potentially make a real contribution in increasing efficacy of drug development pipelines (Figure 8) [6].
Recently we succeeded to find in a C57bl6 mouse modelusing similar LCMS techniques using a lipidomics based approach-in liver homogenates of the 24 h starvation group a 49:4-TG with an odd number of C atoms appeared which was not present in the control group [19]. Odd triacylglycerols are rare and can possibly be used as biomarkers in case of starvation for “functional foods” [9] or in case of starvation [19].
In contrast in this research manuscript with a C57Bl6 highfat diet obese induced mouse model we found two potential biomarkers of two different lipid fractions for type 2 diabetes (T2DM): a 20:3 cholesteryl ester (ChE) and a 36:1 Phosphatidylcholine (PC) in hearth muscle [21]. Interestingly the 20:3 ChE biomarker has also been found and classified as a potential T2DM biomarker in a human cohort of the Uppsala longitudinal study of adult men (ULSAM). This is a cohort which includes 2,322 50-year old mean who have been followed for 35 years with detailed measurements every 10 years and classified as dihomo-γ-linolenic (20:3 n-6) [32]. With respect to this 20:3 ChE one of the most interesting yet controversial dietary approaches has been the possible prophylactic role of dietary γ-linolenic acid (GLA) in treating various chronic disease states. This strategy is based on the ability of diet to modify cellular lipid composition and eicosanoid (cyclooxygenase and lipoxygenase) biosynthesis (Figure 9). Recent studies demonstrate that dietary GLA increases the content of its elongase product, dihomo-γ- linolenic acid (DGLA), within cell membranes without concomitant changes in arachidonic acid (AA). Subsequently, upon stimulation, DGLA can be converted by inflammatory cells to 15-(S)-hydroxy-8,11,13-eicosatrienoic acid and prostaglandin E1 (Figure 10).
This is noteworthy because these compounds possess both anti-inflammatory and anti-proliferative properties [33]. Most important EFA is gamma-linolenic acid (GLA)-18 carbons, onecarboxylic, non-branched fatty acid with 3 double cis-bonds (the last is situated by 6-th carbon from methylic end). Modern research of GLA and others EFA's is concerned mainly on therapeutic impact on the inflammatory process. The biogenic amines, cytokines, prostaglandins, thromboxanes and leukotrienes are the main inflammatory mediators. The last three are described with the common name eicosanoids (eicotwenty). Eicosanoids are synthesized from 20-carbon unsaturated fatty acids: dihomo-gamma-linoleic (DGLA) (20:3, cis delta [8,11,14], omega6), arachidonic acid (AA-20:4, cis delta (5,8,11,14), omega6), and eicosapentanoic acid (EPA-20:5, cis delta (5,8,11,14,17, omega3). Derivatives of gamma and gamma-linolenic acids regulate the inflammatory process, through their opposed activity.
PG2, leucotrien C4 and tromboxan A2 have the strongest proinflammatory action. Derivatives of alpha-linolenic acid 15- HETE and prostaglandin E1 (PGE1) have weak proinflammatory action, or even anti-inflammatory (PGE1), and additionally, they inhibit the transformation of arachidonic acid (AA) to leukotriens. delta6-desaturase (transforms linolenic acid into gamma-linolenic acid by making additional double bond) is the slowest step of the fatty acid metabolism. It's activity is impaired by many physiological and pathologic factors and leads to gamma-linolenic acid (GLA) deficiency (Figure 3) [34].
In contrast, the 36:1 PC has until this moment not yet been classified in a human cohort as biomarker for T2DM and/or consequently cardiovascular diseases. Indeed the levels of (poly-unsaturated) fatty acids and lyso-phospholipids may be significantly increased during hypoxia or ischemia [35,36].
A relationship has been reported between the fatty acid composition of phosphatidylcholine (PC) and or Lysophosphatidyl choline (LPC) with PC as membrane phospholipids, its membrane fluidity, and its sensitivity to insulin. PC, (once given the trivial name ‘lecithin’) is usually the most abundant phospholipid in animals and plants (up to 50%) and is as such obviously the key building block of membrane lipid bilayers [30]. Changes in the concentrations of PC may affect the properties of the membrane lipid bilayer. Phosphatidylcholine can be converted by enzymatic activity of phospholipase A1 towards lysophosphatidyl choline in the “lands cycle” [30] (Figure 11).
Very important, in this context, is to remark that cardiovascular disease are one of the major complication of T2DM resulting in 17 million deaths a year [37]. For this 36:1 PC biomarker until presently no plausible mechanisms for its formation are mentioned in the literature. We suggest the following biochemical explanation.
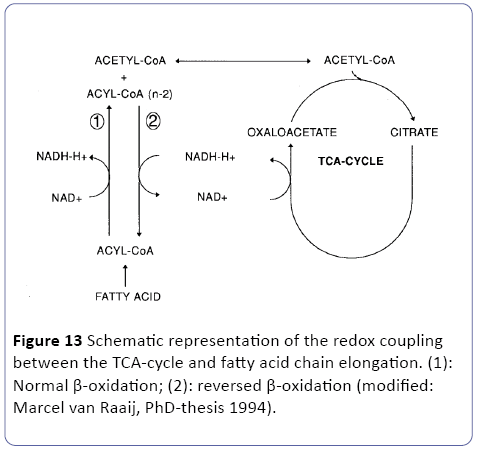
In order to conserve the energy from glucose as ATP, three major metabolic pathways are involved three metabolic pathways are involved in the mitochondria (Figure 12), directly linked to the oxidative phosphorylation outside in the cytosol. The glycolysis, the Krebs-cycle (TAC) and the β-oxidation [23,24]. So our perception is that in white adipose tissue (WAT) low oxygen (hypoxia) are the case as outlined in the research article of and the review of [38,39]. So as stated earlier and depicted in Figure 1 of WAT ischemic and/or hypoxic conditions may occur. Under these conditions, it can be questioned how the TCA cycle persist in its activity and the redox balance maintained. Since the early work at hypoxic conditions at fish clearly reviewed and outlined by van Raaij in his PhD-thesis [40] and recently in tumor research [41,42] two hypothesis has been postulated related to these two research topics: fatty chain elongation and anaerobic endogenous reduction of unsaturated fatty acids [23,24]. We suggest in this overall publication of an obese/type-2 diabetes C57bl6 mouse model the following two mechanisms:
Figure 12: Biochemical overview of the three major pathways in the mitochondria: the glycolysis, the Krebs cycle (TCA) and the β-oxidation. Under hypoxic conditionswhich is the case in excessive WAT-tissue-both the TCAcycle and the β-oxidation needs to maintain their redoxbalance which is on one hand performed lipid synthesis and by “reversed β oxidation” (fatty chain elongation) also leading to fat synthesis => so a vicious circle is observed during severe obesity; modified: [23,24].
A): The concept of anaerobic fatty chain elongation involves the coupling of acetyl-CoA units to fatty acids via a reversal of the β-oxidation. The first three reactions are identical to the β- oxidation. However, the last reaction is catalyzed by the NADdependent enoyl-CoA reductase instead of the Co-Q dependent acetyl-Co-A dehydrogenase. In this way, the reaction is thermodynamically favored in the direction of chain elongation -which corresponds to fat synthesis [43]. Since fatty chain elongation consumes 2 moles of NADH in each cycle, this pathway provides a suitable mechanism to maintain the mitochondrial redox balance [23,24] (Figure 13).
Indeed, fatty acid chain elongation (i.e. lipid synthesis) during anoxia could be stimulated in vitro by addition of TCAcycle intermediates such as glutamine [42] which is indicative for the redox coupling between TCA-cycle activity and fatty acid chain elongation.
Fatty acid chain elongation (i.e. lipid synthesis) during anoxia/ischemia has been demonstrated in various organisms starting with invertebrate models and finding its way towards biomedicine [43,44]. But most important for cardiovascular diseases FA chain elongation has been observed in ischemic arterial tissue [35] and ischemic mammalian heart [45]. In the sections above, the lipid metabolism has been proposed as a suitable mechanism to maintain redox balance in anoxic tolerant invertebrates [44]. However, hypoxia may have also adverse effects on lipid metabolism, resulting in a decreased cellular functional integrity and eventually tissue damage. Especially in mammalian tissues being essentially hypoxia intolerant, these effects have received large attention [17].
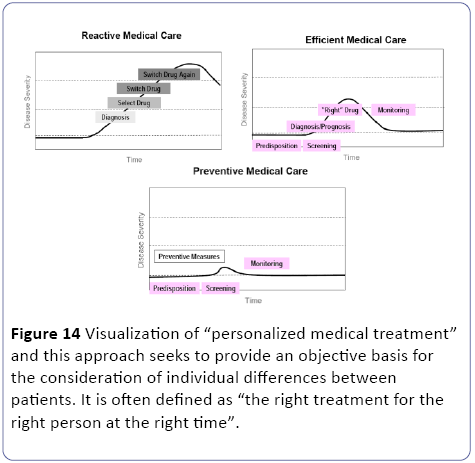
When oxygen availability is decreased, the oxidative phosphorylation is inhibited and as a result of the accumulating reducing equivalents (NADH, FADH2), the fluxes through the TCA-cycle and β-oxidation are impaired. Since the flux through the β-oxidation is high during normoxic conditions [23,24], fatty acids and their metabolites rapidly accumulate during anaerobiosis [46]. In addition, during hypoxia or ischemia, the cellular energy status may be affected. A number of ionic rearrangements occur due to the inhibition of ATPdependent membrane associated ion pumps and intracellular acidosis. This may activate phospholipases which results in a marked hydrolysis of membrane lipids [23,24]. But when an appropriate biomarker for a complex human disease like T2DM is found it can lead to a “personalized medical treatment” [25] were per individual the effects of nutritional intervention exercise protocol or drug therapy should be considered part of a lifestyle strategy to prevent or manage T2DM (Figure 14).
Conflict of interest
The authors declare no conflict of interests.
Acknowledgements:
Experiments were supported by a grant of the Center for Medical Systems Biology (CMSB), Leiden University.
References
- Wishart DS, Knox C, Guo AC, Eisner R, Young N (2009) HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 37: 603-610.
- Ahn AC, Tewari M, Poon CS, Phillips RS (2006) The limits of reductionism in medicine: could systems biology offer an alternative?. PLoS Med 3: 208.
- Rees DA, Alcolado JC (2005) Animal models of diabetes mellitus. Diabet Med 22: 359-370.
- King AJF (2012) The use of animal models in diabetes research, Br J Pharmacol 166: 877-894.
- Ali O (2013) Genetics of type 2 diabetes, World J. Diabetes 4: 114.
- Laaksonen R, Jänis MT, Orešic M (2008) Lipidomics-based safety biomarkers for lipid-lowering treatments, Angiology 59: 65-68.
- Kitano H (2002) Systems biology: a brief overview, Science 295: 1662-1664.
- van der Greef J, Hankemeier T, McBurney RN (2006) Metabolomics-based systems biology and personalized medicine: moving towards n = 1 clinical trials?, Pharmacogenomics 7: 1087-1094.
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, et al. (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345 (2001) 790-797.
- Blackburn GL (2001) Pasteur’s Quadrant and malnutrition. Nature 409: 397-401.
- Arab L (2003) Biomarkers of fat and fatty acid intake, J Nutr 133: 925-932.
- Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L (2005) Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 162: 373-381.
- van der Greef J, Davidov E, Verheij E, Vogels E, Adourian AS (2003) The role of metabolomics in systems biology, in metabolism profiling its role Biomark Discov Gene Funct Anal, Springer pp: 171-198.
- van der Greef J, van der Heijden R, Verheij ER (2004) The Role of Mass Spectro-metry in Systems Biology: Data Processing and Identification Strategies in Metabolo-mics. In: Ashcroft GBAE, Monaghan JJ (Eds.), Adv Mass Spectrom, Amsterdam: Elsevier Science.
- Meikle P, Barlow C, Weir J (2009) Lipidomics and lipid biomarker discovery. Aust Biochem 40: 12-16.
- Lee YH, Mottillo EP, Granneman JG (2014) Adipose tissue plasticity from WAT to BAT and in between, Biochim. Biophys. Acta-Mol Basis Dis 1842: 358-369.
- Hochachka PW, Lutz PL, Sick TJ, Rosenthal M (1993) Surviving hypoxia: Mechanisms of control and adaptation, CRC Press.
- Christie WW (1989) Gas chromatography and lipids.
- Van Ginneken V, Verhey E, Poelmann R, Ramakers R, van Dijk KW, et al. (2007) Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study of hepatic steatosis, Biochim. Biophys. Acta (BBA)-Molecular Cell Biol. Lipids 1771: 1263-1270.
- van Ginneken VJT, Feskens E, Poelmann RE (2010) Review: Insulin Resistance: Intra-uterine growth retardation, life style, genetic susceptibility, prevention and treatment., in: L. V Berhardt (Ed.), Adv Med Biol Res, Nova Science Publishers.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2016) Metabolomics in Hind limb and Heart Muscle of a Mouse Model after a High-fat Diet, Anat Physiol 6: 940-2161.
- van Woudenbergh GJ, Kuijsten A, Sijbrands EJG, Hofman A, Witteman J (2011) Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab.
- Salway JG (2004) others, Metabolism at a glance. Metab a Glance.
- Salway JG (2006) Medical Biochemistry At a Glance (Ed.), Blackwell publishing ltd.
- van der Greef J, Hankemeier T, McBurney RN (2006) Metabolomics-based systems biology and personalized medicine: moving towards n= 1 clinical trials?, Pharmacogenomics 7: 1087.
- Jaffrézou JP, Levade T, Bettaïeb A, Andrieu N, Bezombes C (1996) Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J 15: 2417-2424.
- Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, et al. (2004) Relationship between insulin Sensitivity and Sphingomyelin Signaling Pathway in Human Skeletal Muscle, Diabetes 53: 1215-1221.
- Marggraf WD, Anderer FA, Kanfer JN (1981) The formation of sphingomyelin from phosphatidylcholine in plasma membrane preparations from mouse fibroblasts, Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 664: 61-73.
- Field A (2005) Discovering Statistics Using SPSS.
- Christie (2003) Lipid Analysis-Isolation, Separation, Identification and Structural Analysis of Lipids.
- Ramautar R, Berger R, van der Greef J, Hankemeier T (2013) Human metabolomics: Strategies to understand biology. Curr Opin Chem Biol 17: 841-846.
- Risérus U, Willett WC, Hu FB (2009) Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 48: 44-51.
- Fan YY, Chapkin RS (1998) Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr 128: 1411-1414
- Wang X, Lin H, Gu Y (2012) Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis 11: 25.
- Ander BP, Dupasquier CM, Prociuk MA, Pierce GN (2003) Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol 8: 164-72.
- Buckler, Honoré E (2005) The lipid-activated two-pore domain K+ channel TREK-1 is resistant to hypoxia: implication for ischaemic neuroprotection. J Physiol 562: 213-222.
- Diabetes Atlas (2014) 6th edition, IDF.
- Trayhurn P, Alomar SY (Oxygen deprivation and the cellular response to hypoxia in adipocytes-Perspectives on white and brown adipose tissues in obesity, Front. Endocrinol.
- Gatterer NH, Faulhaber M, Burtscher M, Pramsohler S, Pesta D (2015) Hypoxia, Oxidative Stress and Fat, Biomolecules 5: 1143-1150.
- Raaij MTM (1994) Lipid metabolism in teleost fish and the hormonal control of substrate mobilization during hypoxia and anoxia, phdthesis, [Sl]: Van Raaij.
- Baenke F, Peck B, Miess H, Schulze A (2013) Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development., Dis. Model. Mech. 6: 1353-1363.
- Sun RC, Denko NC (2014) Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth, Cell Metab 19: 285-292.
- Seubert W, Podack ER (1973) Mechanisms and physiological roles of fatty acid chain elongation in microsomes and mitochondria. Mol Cell Biochem 1: 29-40.
- Bulla LA, Cheng TC (2013) Invertebrate models for biomedical research, Springer Science & Business Media.
- Lopaschuk GD, Belke DD, Gamble J, Toshiyuki I, Schönekess BO (1994) Regulation of fatty acid oxidation in the mammalian heart in health and disease, Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1213: 263-276.
- Morash AO, Kotwica AJ, Murray (2013) Tissue-specific changes in fatty acid oxidation in hypoxic heart and skeletal muscle. Am J Physiol Regul Integr Comp Physiol 305: 534-541.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences