Potential Mechanisms Underlying TGF-ÃÆà ½Ãâò-mediated Complement Activation in Lung Fibrosis
Amanda J Fisher, Ellyse Cipolla, Ananya Varre, Hongmei Gu, Elizabeth A Mickler and Ragini Vittal
DOI10.21767/2573-5365.100037
1Department of Medicine, Division of Pulmonary and Critical Care Medicine, Indiana University School of Medicine, Indianapolis, Indiana
2Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor, USA
- *Corresponding Author:
- Ragini Vittal
Assistant Research Professor
Department of Medicine, Division of Pulmonary and Critical Care Medicine
University of Michigan, Ann Arbor, MI 48109, USA.
Tel: 734-763-4359
E-mail: rvittal@med.umich.edu
Received Date: October 18, 2017; Accepted Date: November 15, 2017; Published Date: November 22, 2017
Citation: Fisher AJ, Cipolla E, Varre A, Gu H, Mickler EA, et al. (2017) Potential Mechanisms Underlying TGF-β-mediated Complement Activation in Lung Fibrosis. Cell Mol Med. Vol.3 No.2:14 DOI: 10.21767/2573-5365.100037
Abstract
While our previous studies suggest that limiting bleomycin-induced complement activation suppresses TGF-β signaling, the specific hierarchical interactions between TGF-β and complement in lung fibrosis are unclear. Herein, we investigated the mechanisms underlying TGF-β-induced complement activation in the pathogenesis of lung fibrosis. C57-BL6 mice were given intratracheal instillations of adenoviral vectors overexpressing TGF-β (Ad-TGFβ) or the firefly gene-luciferase (Ad-Luc; control). Two weeks later, mice with fibrotic lungs were instilled RNAi specific to receptors for C3a or C5a -C3ar or C5ar, and sacrificed at day 28. Histopathological analyses revealed that genetic silencing of C3aR or C5ar arrested the progression of TGF-β-induced lung fibrosis, collagen deposition and content (hydroxyproline, Col1a1/2); and significantly suppressed local complement activation. With genetic silencing of either C3aR or C5ar, in Ad-TGFβ-injured lungs: we detected the recovery of Smad7 (TGF-β inhibitor) and diminished local release of DAF (membrane-bound complement inhibitor); in vitro : TGF-β-mediated loss of DAF was prevented. Conversely, blockade of the TGF-β receptor prevented C3a -mediated loss of DAF in both normal primary human alveolar and small airway epithelial cells. Of the 52 miRNAs analyzed as part of the Affymetrix array, normal primary human SAECs exposed to C3a , C5a or TGF-β caused discrete and overlapping miRNA regulation related to epithelial proliferation or apoptosis (miR- 891A, miR-4442, miR-548, miR-4633), cellular contractility (miR-1197) and lung fibrosis (miR-21, miR-200C, miR-31HG, miR-503). Our studies present potential mechanisms by which TGF-β activates complement and promotes lung fibrosis.
Keywords
C3aR, C5ar, DAF, Smad7, miR-21, miR-200C
Abbreviations
Ad-Luc: Adenoviral Vector Expressing the Firefly Gene-Luciferase; Ad-TGFβ: Adenoviral Vector Overexpressing Transforming Growth Factor Beta; C3a : Complement component 3a; C5a : Complement component 5a; C3aR: C3a Receptor; C5ar: C5a Receptor; Col(I): Collagen Type I; DAF: Decay Accelerating Factor; GADPH: Glyceraldehyde-3-Phosphate Dehydrogenase; PBS: Phosphate Buffered Saline; IT: Intratracheal; IP: Intraperitoneal; Col1a1: Collagen Type I Alpha 1 Chain; Col1a2: Collagen Type I Alpha 2 Chain; H&E: Hematoxylin and Eosin; BALF: Bronchoalveolar Lavage Fluid; C5b-9: Terminal Complement Complex; IPF: Idiopathic Pulmonary Fibrosis; IUSM: Indiana University School of Medicine; PCR: Polymerase Chain Reaction; RNA: Ribonucleic Acid; qPCR: Quantitative (Real-Time) Polymerase Chain Reaction; siRNA: Small Interference Ribonucleic Acid; φ siRNA: Non- Targeting siRNA; TGFβ1: Transforming Growth Factor Beta Isoform 1; PFU: Plaque Forming Units; AU: Arbitrary Units; RQ: Relative Quantification; FDR: False Discovery Rate; C3aRA: C3a Receptor Antagonist; ALK5 In: Alkivin-like 5 Inhibitor
Introduction
Idiopathic Pulmonary Fibrosis (IPF) is a devastating disease characterized by progressive scarring of the lung. The architectural remodeling has long been debated as a process involving multiple alveolar epithelial injuries which triggers a number of chronic inflammatory signals which in turn drives dysregulated tissue repair. TGF-β has emerged as the undisputed master regulator of fibrosis which triggers a number of other pro-fibrotic signals which serve as a feed-forward loop and augment lung remodeling. Additionally, a number of other inflammatory signals trigger the autocrine synthesis of TGF-β in the lung. Studies from our lab demonstrate two different inflammatory signaling cascades, i.e. IL-17A [1] and the complement cascade [2] which trigger TGF- β-related signaling pathways. Our recent report also suggests that IL-17A may drive fibrosis via the complement cascade [3]. Signaling interactions between these pro-fibrotic pathways may shed significant insights on the specific drivers of epithelial injury and dysregulated tissue repair.
The complement cascade has been implicated in IPF [2,4-6] as well as several other autoimmune diseases, including chronic lung allograft rejection [7], renal transplant [8] and allergic asthma [9]. The complement cascade is an integral arm of innate immunity with the components C3a and C5a being the major players in epithelial injury. Studies have also implicated a role for microRNAs in epithelial injury [10] and TGF-β-driven lung fibrosis [11-14]. MicroRNAs are post-transcriptional gene regulators that function by binding to specific sequences, typically in the 39-untranslated region of the target mRNAs and blocking translation or causing the rapid degradation of the target transcript [15]. Interestingly, studies that have been reported on C3a or C5a -driven miRNA regulation in causing epithelial injury or lung fibrosis are lacking.
In this study, we examined the hierarchical relationship of TGF-β and the complement cascade in driving the pathogenesis of lung fibrosis. In our prior report [4], we had shown that limiting complement activation by blocking the receptors for C3a or C5a attenuates systemic TGF-β activity and local TGF-β- related transcriptional signaling. Therefore, it is not known if the complement cascade is upstream of TGF-β. We thus hypothesized that if the complement cascade is upstream then there will not be any protection against direct TGF-β-induced lung fibrosis. However, if there is some protection then that would indicate that these pathways are parallel and share mechanisms that drive lung fibrosis. To address these questions, we utilized the adenoviral vector-mediated overexpression of TGF-β in order to induce lung fibrosis and blocked the complement cascade therapeutically using siRNA sequences specific to C3aR and C5ar. We then performed a series of experiments utilizing the murine lung tissues from our murine model and normal primary human airway and alveolar epithelial cells to further address mechanisms in vitro .
Materials and Methods
Cell culture conditions and reagents
Normal primary human alveolar type II epithelial cells (hAECs; Cell Biologics, Chicago, IL) were grown in human alveolar epithelial basal media supplemented with serum and growth factors (Cell Biologics). The cells were seeded at 70% confluence and incubated in 5% CO2-95% air. Before stimulation, cells were growth arrested using basal media alone for 1 h. Normal primary human small airway epithelial cells (SAECs; Clonetics, Cambrex Biosciences, Walkersville, MD) from five different donor lungs were grown in small airway basal media supplemented with growth factors (Clonetics). Before stimulation, cells were growth arrested using 0.01% serum or 1:100 growth factors containing media for 1h. These studies used recombinant human C3a and C5a (100 nM; Complement Technology, Inc., Tyler, TX), platelet-derived TGF-β1 (2 ng/ml; Roche Diagnostics, Germany). Antagonists against C3aR (2.5 μM; C3aRA-SB290157) and ALK5 (2.5 μM; ALK5 In-SB431542) were purchased from Calbiochem, EMD Millipore, Billerica, MA. All other reagents were from Sigma.
Animal studies
The Animal Care and Use Committee at the Indiana University School of Medicine approved the animal protocols used in this study. C57-BL6 mice (female, 8 weeks; Jackson Laboratories, Bar Harbor, ME) were anesthetized by intraperitoneal injections of a mixture of ketamine hydrochloride (10 mg/kg) and xylazine (150 mg/kg). After adequate anaesthesia, approximately 40 μl of suspension containing 1 × 109 PFU of adenoviral vectors overexpressing TGF-β1 (ViraQuest, North Liberty, IA) or the firefly gene-luciferase (Welgen Inc., Worcester, MA) [1] were delivered intratracheally. The mice were placed under observation until they recovered consciousness. Mice were monitored daily for adverse reactions and changes in behaviour.
RNA interference (RNAi) studies
For in vivo oropharyngeal RNAi delivery, single-duplex small interference RNA (siRNA) sequences targeting C3aR and C5ar [4] (50 μg; Sigma), or non-targeting control siRNA (50 μg; Dharmacon Technologies, Pittsburgh, PA) were used. For in vitro RNAi transfection in hSAECs, single duplexes siRNA sequence targeting C3aR or C5ar or non-targeting control siRNA (100 nM; Sigma) were transfected using Oligofectamine (Invitrogen, Foster City, CA) for 24 h. Subsequently, the transfected cells were cultured in basal media with 1:100 growth factors for 16 h followed by treatment.
Western blotting
Cell lysates of primary normal human small airway epithelial cells (hSAECs) and alveolar type II epithelial cells (hAECs) and acellular BALF were analyzed for equal protein concentrations and then subjected to immunoblotting as previously described [1-4,16,17]. Antibodies used were against C3aR [3], and C5ar [3] were purchased from Novus Biologicals, Littleton, CO. CD46 [2] and DAF [3] antibodies were purchased from Santa Cruz Biotechnology. Densitometric analyses were performed with ImageJ 1.32j (NIH, Bethesda, MD).
Hydroxyproline content of whole lung
We homogenized mouse whole lungs in PBS and then acidified (by adding an equal volume of 12 N HCl), hydrolyzed (by heating at 120°C for 24 h), and processed samples for hydroxyproline measurements as previously described [18].
Real-time polymerase chain reaction (qPCR)
Total RNA was isolated from cells and whole lung homogenates with the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed with qScript cDNA SuperMix (Quanta BioSciences Inc., Foster City, CA). Real-time PCR was performed for each cDNA using Taqman Assays (Applied Biosystems, Inc., Foster City, CA). The semi-quantitative real-time PCR data for each target gene are expressed as 2-Ct relative quantitation vs. endogenous control, with error bars representing the standard error.
ELISA
Acellular BALF derived from mice treated with the siRNA-specific to C3aR or C5ar, were used to measure the soluble form of C5b-9 using Terminal Complement Complex C5b9 BioassayTM ELISA kit (mouse), (US Biological Lifesciences Inc., Salem, MA), as per manufacturer’s protocol. The active forms of C3a and C5a were measured in the BALF using Mouse complement fragment 3a ELISA kit and Mouse complement fragment 5a ELISA kit (MyBiosource, San Diego, CA) respectively, as per manufacturer’s protocol.
Affymetrix analysis
SAECs derived from five different normal lungs were exposed to C3a or C5a (100 nM) or TGF-β1 (2 ng/ml) for 24 h. RNA was isolated, and the quality of the total RNA was verified by the Nanodrop (Fisher Scientific, Inc.) by measuring the 260/280 absorbance ratio and confirming that this ratio is at 1.7 and above. cDNA was subjected to Affymetrix analysis by the Microarray Core, Indiana University School of Medicine to perform gene expression profile analysis on Affymetrix HG-U133 Plus 2.0 Arrays, following the manufacturer’s recommendations. Differentially expressed genes were then identified with the rank product method. This method, based on the statistical analysis of the expression rank of each gene in the replicate experiments, has been shown [6] to perform especially well for heterogeneous donor lung derived data and low number of replicates. The method produces an estimate of the false discovery rate (FDR) based on a randomization procedure. Differential expression is defined by the statistical analysis (false discovery rate <0.01) by fold-ratio comparing the agonists to the control in each of the five different normal cells used and the fold change was set at ± 1.5. These statistical analyses were performed by the core facility.
Statistical analysis
Statistical analysis was performed using Student’s t test and oneway ANOVA with Bonferroni as the post hoc test using GraphPad Prism version 4.03 for Windows, GraphPad Software (San Diego, CA, www.graphpad.com); unless otherwise stated. Statistical significance was defined at ‘p’<0.05.
Results
RNA-interference specific to C3aR or C5ar arrests the progression of TGF-β-induced lung fibrosis
We had reported elevated local C3a and C5a in clinical [2] and experimental lung fibrosis [4]. Our prior studies indicate an induction of C3aR and C5ar expression in lung epithelial cells in response to TGF-β [2]; and in mesenchymal cells in response to C3a or C5a [4]. We also reported that C3a and C5a each induce mRNA expression of TGF-β and suppress that of Smad7 [2]. Further, blockade of C3aR and C5ar expression mitigated the progression of bleomycin-induced lung fibrosis by suppressing local TGF-β-related signaling and systemic TGF-β activity [4]. We next sought to determine whether this reduction in TGF-β activity is responsible for the protection, or simply an arrest in bleomycin-induced lung fibrosis lowers TGF-β activity and signaling. We hypothesized that if genetic silencing of C3aR or C5ar arrests the progression of lung fibrosis via the reduction in systemic TGF-β activity and local TGF-β-related mRNA expression, then this protection should be reversed in fibrotic environment driven by TGF-β. Towards this end, we employed an adenoviral vector overexpressing TGF-β in mice. Adenoviral overexpression of TGF-β has induced lung fibrosis in monkeys [19] and wildtype mice [20,21] but not in Smad3 knockout mice [22]. In the current report, we subjected wildtype mice to lung injury by intratracheal administration of adenoviral vectors expressing the firefly geneluciferase( Ad-Luc) or overexpressing TGF-β (Ad-TGF-β).
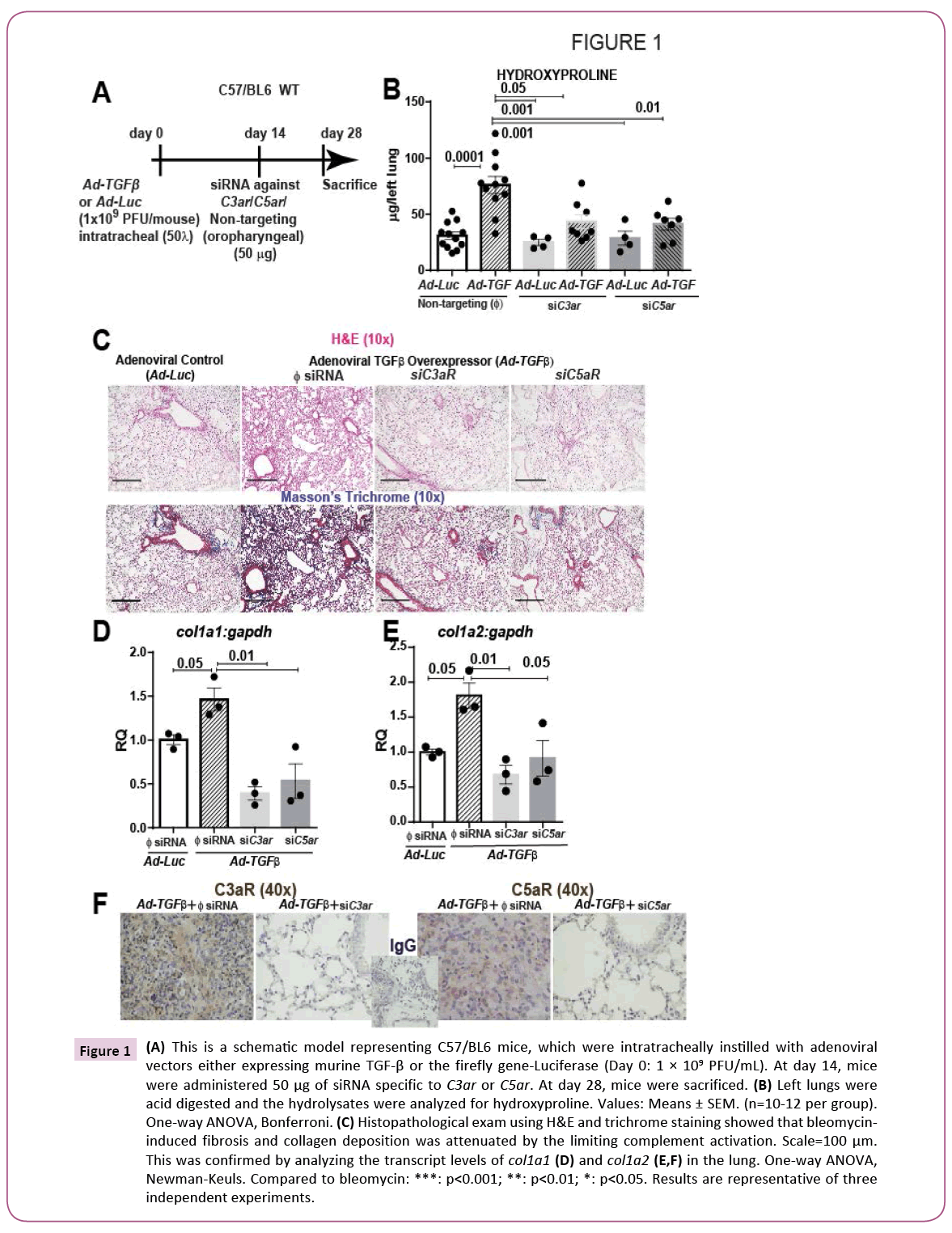
We then mimicked the clinical setting wherein IPF patients present themselves with lung fibrosis and used a therapeutic strategy (Figures 1A-1F) as reported previously [3,4,16,17]. At two weeks after the onset of fibrosis, we then silenced the gene expression of C3aR or C5ar using oropharyngeal instillation of naked siRNA. Histopathological examination of mice exposed to Ad-TGF-β revealed significant fibrosis (Figure 1C; upper panels) and collagen deposition as shown by Masson’s trichrome staining (Figure 1C; lower panels). Further, quantitative analysis of collagen content was significantly higher as shown by hydroxyproline levels (Figure 1B) and mRNA expression of Col1a1 and Col1a2 (Figure 1D). Interestingly, Ad-TGF-β-injured mice that received siRNA specific to either C3aR or C5ar showed a near normal lung architecture and significantly lower collagen deposition and collagen content. Collectively, our results show a protection against TGF-β-induced lung fibrosis with blockade of C3a or C5a signaling on binding to their respective receptors. In Figure 1E, we show that the siRNA specific to C3aR and C5ar1 instilled into these lungs injured with the lentiviral bvectors overexpressing TGF-β, were indeed effective in silencing the C3aR and C5ar protein expressions. These data suggest that either the two pathways are triggered in parallel or that TGF-β is upstream of signaling due to C3a or C5a .
Figure 1: (A) This is a schematic model representing C57/BL6 mice, which were intratracheally instilled with adenoviral vectors either expressing murine TGF-β or the firefly gene-Luciferase (Day 0: 1 × 109 PFU/mL). At day 14, mice were administered 50 μg of siRNA specific to C3aR or C5ar. At day 28, mice were sacrificed. (B) Left lungs were acid digested and the hydrolysates were analyzed for hydroxyproline. Values: Means ± SEM. (n=10-12 per group). One-way ANOVA, Bonferroni. (C) Histopathological exam using H&E and trichrome staining showed that bleomycininduced fibrosis and collagen deposition was attenuated by the limiting complement activation. Scale=100 μm. This was confirmed by analyzing the transcript levels of Col1a1 (D) and Col1a2 (E,F) in the lung. One-way ANOVA, Newman-Keuls. Compared to bleomycin: ***: p<0.001; **: p<0.01; *: p<0.05. Results are representative of three independent experiments.
RNA interference suppresses local complement activation
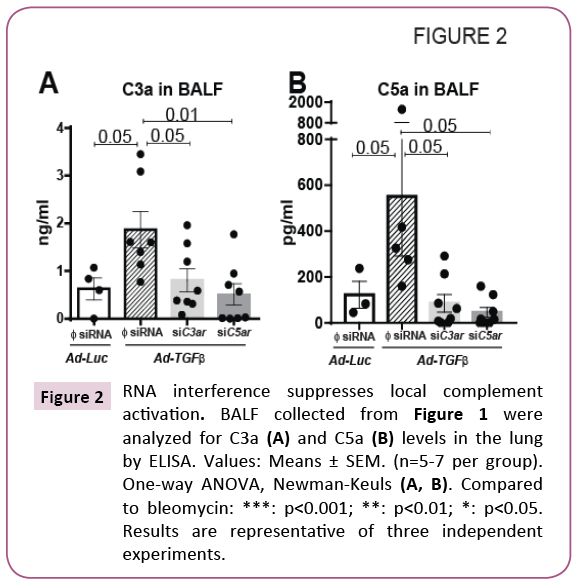
We have previously demonstrated that local C3a and C5a levels are higher due to bleomycin injury at day 14 [4] and that genetic silencing of their receptors suppressed bleomycin-induced local C3a , C5a and soluble C5b-9 levels [4]. We have also observed a suppression of these complement components due to bleomycin injury in IL-17A deficient mice [3]. Furthermore, we had reported that IL-17A-mediates epithelial injury via TGF-β during bronchiolitis obliterans [1]. We next sought to define the hierarchical role of C3a and C5a on binding to their respective receptors in a TGF-β-induced murine model of lung fibrosis. Since IPF patients present with established fibrosis, we simulated the clinical condition and examined the effects of targeted genetic silencing of C3aR and C5ar in a significantly scarred lung. Using the treatment regimen shown in Figure 1A, the mice were subjected to oropharyngeal instillation of siRNA specific to C3aR and C5ar at day 14-a time point when the lungs are significantly fibrotic. Increased levels of C3a and C5a in the BALF were reported in transfusion-related acute lung injury [23] and chronic rejection post-lung transplant [24]. Elevated tissue deposition of C5b9 was reported during acute rejection phase post-lung transplant [25]. Our studies show that active C3a and C5a levels were higher in the BALF of mice exposed to adenoviral vectors overexpressing TGF-β. However, genetic silencing of C3aR or C5ar suppressed the local levels of C3a and C5a as shown in Figures 2A and 2B. These results suggest that TGF-β-induced activation of the complement cascade is attenuated by diminishing the expression of the receptors for C3a and C5a possibly via a feedback loop.
Figure 2: RNA interference suppresses local complement activation. BALF collected from Figure 1 were analyzed for C3a (A) and C5a (B) levels in the lung by ELISA. Values: Means ± SEM. (n=5-7 per group). One-way ANOVA, Newman-Keuls (A, B). Compared to bleomycin: ***: p<0.001; **: p<0.01; *: p<0.05. Results are representative of three independent experiments.
Potential mechanisms underlying TGF-β-induced complement activation
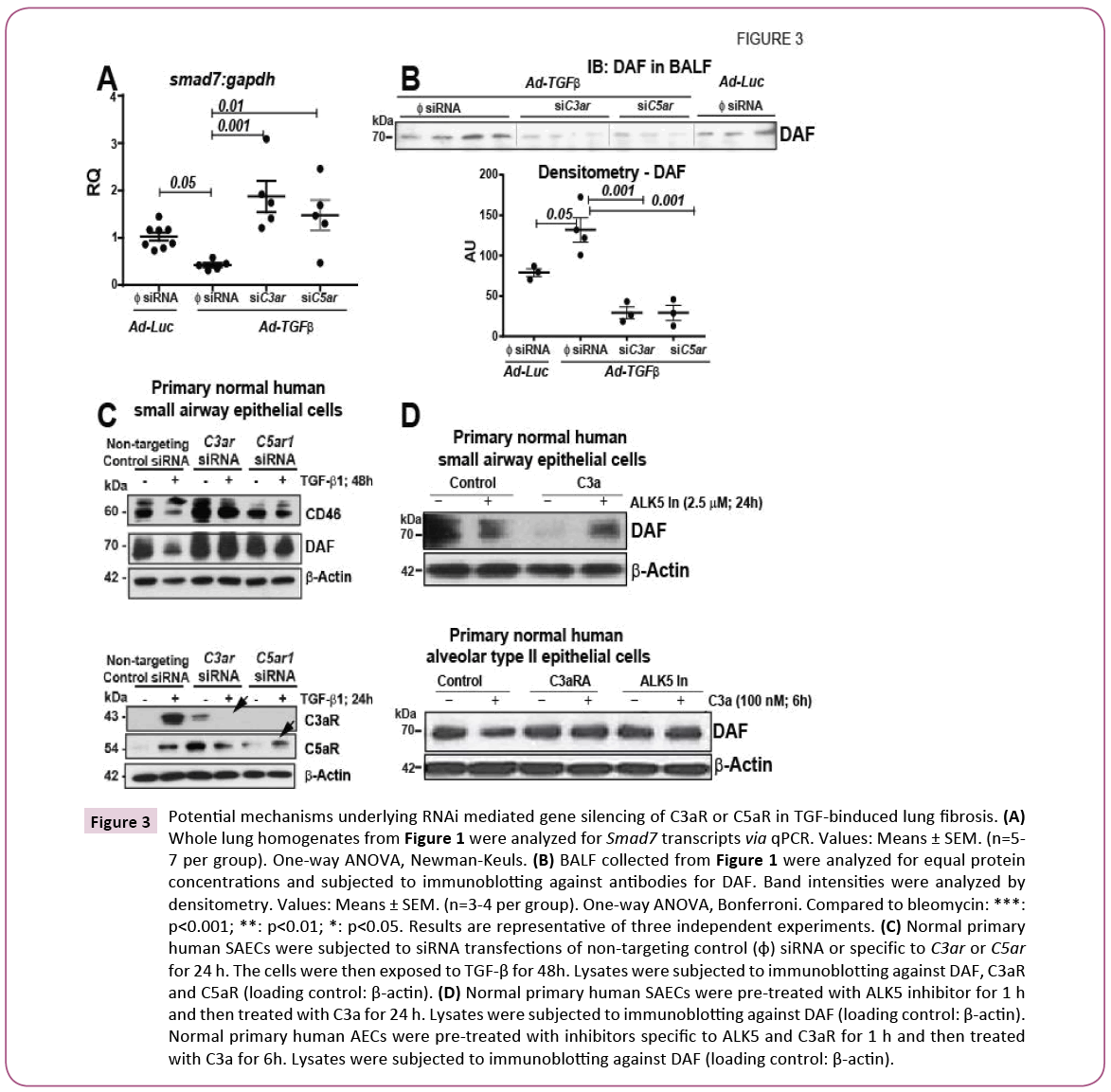
We next investigated the potential mechanisms underlying TGF- β-induced complement activation in response to therapeutic gene silencing of C3aR or C5ar. We had previously reported that C3a and C5a each downregulate the mRNA expression of Smad7 and that TGF-β upregulated the protein expressions of both C3aR and C5ar in primary normal human SAECs [2]. We therefore hypothesized that siRNA specific genetic silencing of C3aR or C5ar will prevent C3a or C5a -mediated downregulation of Smad7 expression. Figure 3A shows a significant recovery of TGF-β- induced downregulation of Smad7 mRNA expression in fibrotic murine lungs due to genetic silencing of C3aR or C5ar. Our prior reports show that TGF-β mediates the loss of membrane-bound DAF from SAECs. Further, we had reported that loss of DAF leads to epithelial apoptosis [2]. Since DAF is a GPI-anchored membranebound molecule, we hypothesized that genetic silencing of C3aR or C5ar may stem the extent of cleavage or the release of DAF onto the lumen of the alveoli and the airways. In our studies, we subjected acellular BALF of equal protein concentrations to immunoblotting (Figure 3B) and detected highest amount of DAF cleavage or release in TGF-β-overexpressing lungs. This release was significantly suppressed due to genetic silencing of C3aR or C5ar as demonstrated by the densitometric analyses of the band intensity. Our results herein suggest that genetic inhibition of signaling due to C3a or C5a may likely protect against TGF- β-induced lung fibrosis by preventing further downregulation of Smad7 due to the additive effects of complement fragments and by preventing epithelial injury due to loss of DAF.
Figure 3: Potential mechanisms underlying RNAi mediated gene silencing of C3aR or C5ar in TGF-binduced lung fibrosis. (A) Whole lung homogenates from Figure 1 were analyzed for Smad7 transcripts via qPCR. Values: Means ± SEM. (n=5- 7 per group). One-way ANOVA, Newman-Keuls. (B) BALF collected from Figure 1 were analyzed for equal protein concentrations and subjected to immunoblotting against antibodies for DAF. Band intensities were analyzed by densitometry. Values: Means ± SEM. (n=3-4 per group). One-way ANOVA, Bonferroni. Compared to bleomycin: ***: p<0.001; **: p<0.01; *: p<0.05. Results are representative of three independent experiments. (C) Normal primary human SAECs were subjected to siRNA transfections of non-targeting control (φ) siRNA or specific to C3aR or C5ar for 24 h. The cells were then exposed to TGF-β for 48h. Lysates were subjected to immunoblotting against DAF, C3aR and C5ar (loading control: β-actin). (D) Normal primary human SAECs were pre-treated with ALK5 inhibitor for 1 h and then treated with C3a for 24 h. Lysates were subjected to immunoblotting against DAF (loading control: β-actin). Normal primary human AECs were pre-treated with inhibitors specific to ALK5 and C3aR for 1 h and then treated with C3a for 6h. Lysates were subjected to immunoblotting against DAF (loading control: β-actin).
We had previously reported that pharmacologic blockade of C3aR and C5ar prevents the loss of the complement inhibitors- CD46 and DAF [2]. In this report, in Figure 3C we show that indeed with genetic silencing of C3aR or C5ar, we are able to recapitulate this effect in primary normal human SAECs. We also show effectiveness and specificity of the siRNA by showing the lack of protein expression of the respective receptor. Since we had previously shown that C3a specifically had upregulated the mRNA expression of TGF-β, we next asked the question if C3a causes loss of DAF directly or by indirectly by inducing the release of TGF-β and hence mediate the cleavage or release of DAF. Towards this end, in Figure 3D, we show that pharmacologic blockade the tyrosine kinase of the receptor type I for TGF-β, i.e. ALK5, effectively prevents the loss of membrane-bound cellular DAF at 24 h. We next investigated this effect in normal primary human alveolar type II epithelial cells (hAECs). Our prior studies show that IL-17A mediates loss of cellular DAF within 4 h in hAECs [3]. We therefore investigated the loss of cellular DAF with blockade of receptors of C3a (C3aRA) and TGF-β (ALK5 In) in parallel to assess the extent of protection. We hypothesize that C3a -mediated loss of DAF will be prevented to a higher extent by blocking C3aR since signaling due to C3a and TGF-β is suppressed. The lower panels in Figure 3D show that both “C3aRA” and “ALK5 In” have similar efficacies in preventing C3a -mediated loss of DAF. Thus, our in vitro studies suggest that while it is likely that C3a -mediated loss of DAF may be dependent on TGF-β, they may also be functioning via parallel pathways. Collectively, our in vivo and in vitro observations indicate that TGF-β-induced lung fibrosis activates complement and this is blocked by recovering the expression levels of the inhibitors of either pathways, i.e. SMAD7 and DAF. It is also likely that TGF-β may be upstream of complement and that complement may function in parallel to TGF-β signaling pathway.
Relative miRNA regulation by TGF-β, C3a or C5a in normal primary human small airway epithelial cells
Key regulatory roles for miRNA either due to upregulation of miRNAs including miR-21 [14], miR-154, miR-134, miR-299- 5p, miR-410, miR-382, miR-409-3p, miR-487b, miR-31, and miR-127 [26], miR-145 [27], miR-199-5p [13], or due to the downregulation of miRNAs including miR-let-7d [15], miR-29 [11], miR-200a/b/c [28], miR-17~92 [29], have been detected in IPF lungs or have been implicated in the pathogenesis of lung fibrosis. While most of the miRNA regulation is reported based on TGF-β studies, very few are reported in response to C3a [30] or C5a [31] in neuropathy. Therefore, we asked the question if there are any overlaps in the miRNA regulation due to TGF-β, C3a or C5a and thus yield possible post-transcriptional targets related to epithelial functions or injury and those related to lung fibrosis. We cultured normal primary human SAECs to 70% confluence and exposed them to exogenous doses of TGF-β or C3a or C5a in parallel for 24 h. We used SAECs from at least five different donors to yield biological replicates. We then isolated RNA from the treated cells and subjected them to Affymetrix array analyses. Of the complete human genome, the chip presented a regulation profile of 52 miRNAs. We used a cut-off fold-change value of ~± 1.35 for the regulated miRNAs. From our analyses of the three agonists, we observed changes in 33 miRNAs due to at least one of the agonists and this is presented in Table 1. In response to TGF-β, a total of 27 miRNAs were modulated with upregulation of 18 miRNAs and downregulation of nine miRNAs. In response to C3a , a total of four miRNAs were modulated with upregulation of two miRNAs and downregulation of two miRNAs. In response to C5a , a total of three miRNAs were modulated and all of them were downregulated. It should be noted that miR-548AC was downregulated by both C3a and TGF-β and is implicated in tumor biology. C5a shares a common miRNA with TGF-β: downregulation of miRNA-200C, which is implicated in lung fibrosis, albeit our studies do not show a significant fold change. Interestingly, in response to TGF-β, three (miR-21, miR- 31HG, miR-503) of the 27 upregulated miRNAs are reportedly implicated in the pathogenesis of lung fibrosis. Fold changes in the other miRNAs are presented in Table 1. Our data suggest possible overlap in post-transcriptional regulation of complement with TGF-β in the pathogenesis of lung fibrosis.
| Affymetrix Transcript Cluster ID | Gene Symbol | p-value (C3a) |
FDR (C3a) |
Fold (C3a/Control) |
p-value (C5a) |
FDR (C5a) |
Fold (C5a/Control) |
p-value (TGF-β) |
FDR (TGF-β) |
Fold (TGF-β /Control) |
|---|---|---|---|---|---|---|---|---|---|---|
| 16978624 | MIR548AC | 2.06E-01 | 9.99E-01 | -1.5 | 3.41E-01 | 9.37E-01 | 1.3 | 7.92E-03 | 4.54E-02 | -1.6 |
| 16709851 | MIR4681 | 4.33E-02 | 9.99E-01 | 1.5 | 4.59E-01 | 9.37E-01 | 11 | 6.28E-01 | 4.35E-01 | 1.1 |
| 16837158 | MIR634 | 2.11E-01 | 9.99E-01 | 1.5 | 8.03E-01 | 9.40E-01 | 1.1 | 7.77E-01 | 4.87E-01 | -1.1 |
| 16770061 | MIR548X2 | 5.64E-02 | 9.99E-01 | -1.5 | 1.71E-01 | 937E-01 | -1.2 | 813E-01 | 498E-01 | -1.1 |
| 16747661 | MIR200C | 1.93E-01 | 9.99E-01 | -1.1 | 1.50E-01 | 9.37E-01 | -1.5 | 1.12E-01 | 1.76E-01 | -1.3 |
| 17105179 | MIR1321 | 8.85E-01 | 9.99E-01 | 1.0 | 4.93E-02 | 9.37E-01 | -1.5 | 3.16E-01 | 3.03E-01 | -1.1 |
| 17103748 | MIR501 | 5.96E-01 | 9.99E-01 | -1.1 | 3.08E-02 | 9.37E-01 | -1.5 | 9.31E-01 | 5.32E-01 | -1.0 |
| 16705437 | MIR1254-1 | 1.96E-02 | 9.99E-01 | 0.13 | 7.80E-01 | 9.39E-01 | -1.0 | 1.94E-01 | 2.35E-01 | -1.5 |
| 16788729 | MIR494 | 1.53E-01 | 9.99E-01 | 1.4 | 2.46E -01 | 9.37E-01 | 1.4 | 2.18E-01 | 2.50E-01 | 1.5 |
| 16008521 | MIR4451 | 2.37E-01 | 9.99E-01 | -1.4 | 2.16E-01 | 9.37E-01 | -11 | 3.02E-03 | 3.00E-02 | -1.5 |
| 16872227 | MIR4530 | 1.65E-01 | 9.99E-01 | 12 | 4.43E-01 | 9.37E-01 | 1.1 | 9.50E-02 | 1.61E-01 | 1.5 |
| 16807761 | MIR4310 | 9.75E-01 | 9.99E-01 | -1.0 | 2.91E-01 | 937E-01 | 1.1 | 4.97E-02 | 1.14E-01 | 1.5 |
| 16865054 | MIR520C | 3.07E-01 | 9.99E-01 | 1.1 | 5.14E-02 | 9.37E-01 | 11 | 4.77E-02 | 1.12E-01 | 1.5 |
| 16865104 | MIR519A2 | 9.52E-01 | 9.99E-01 | -1.0 | 1.00E+00 | 9.50E-01 | 1.0 | 2.15E-01 | 2.48E-01 | 1.6 |
| 16734339 | MIR4298 | 2.10E-01 | 9.99E-01 | -1.1 | 9.82E-01 | 9.47E-01 | -1.0 | 2.49E-02 | 7.99E-02 | 1.6 |
| 16735893 | MIR4299 | 4 08E-01 | 9.99E-01 | 12 | 1.91E.01 | 937E.01 | 13 | 9.55E-03 | 5.01E-02 | 1.6 |
| 16836624 | MIR21 | 6.00E.01 | 9.99E-01 | 1.1 | 8.85E-01 | 9.44E.01 | -1.0 | 2.78E-03 | 2.90E-02 | 1.7 |
| 17088997 | MIR18182 | 8.67E-03 | 9.99E.01 | 1.3 | 2.24E-01 | 9.37E-01 | 13. | 3.46E-02 | 9.38E-02 | 1.7 |
| 17002870 | MIR31HG | 6.05E-02 | 9.99E-01 | 1.1 | 3.81E-01 | 9.37E.01 | 1.0 | 1.55E-02 | 6.31E-02 | 1.5 |
| 16988869 | MIR4633 | 395E-01 | 9.99E-01 | 11 | 2.12E-02 | 937E.01 | 1.4 | 2.64E-02 | 8.19E-02 | 1.5 |
| 16664579 | MIR4421 | 9.82E-01 | 9.99E-01 | -1.0 | 3.20E-01 | 937E.01 | 1.1 | 2.31E-01 | 2.58E-01 | 1.5 |
| 16951644 | MIR4442 | 2.82E-01 | 9.99E.01 | 1.1 | 7.32E-01 | 9.37E-01 | 1.0 | 1.63E-02 | 6.46E-02 | 1.5 |
| 16788768 | MIR487A | 415E-01 | 9.99E.01 | -1.0 | 1.52E-01 | 9.37E-01 | 11 | 3.12E-02 | 8.91E-02 | 1.5 |
| 16947780 | MIR551B | 585E.01 | 9 99E-01 | 1.1 | 981E.01 | 9.47E.01 | -1.0 | 5.25E-02 | 1.18E-01 | 1.5 |
| 16788719 | MIR1197 | 632E.01 | 999E-01 | 12 | 8.85E.01 | 9.44E.01 | 10 | 2.36E-01 | 2.61E-01 | 1.5 |
| 17114334 | MIR503 | 8.13E.01 | 9.99E.01 | -1.0 | 7.93E-01 | 9.39E.01 | -1.0 | 5.50E-02 | 1.20E-01 | 1.5 |
| 17114774 | MIR891A | 4.50E-01 | 9.99E.01 | -1.1 | 6.91E-01 | 9.37E-01 | 1.1 | 3.67E-02 | 9.66E-02 | -1.9 |
| 16748449 | MIR1244-1 | 7.02E-01 | 9.99E-01 | -1.0 | 2.08E-02 | 9.37E.01 | 12 | 3.21E-03 | 3.07E-02 | -1.6 |
| 16761116 | MIR1244-1 | 702E-01 | 999E-01 | -10 | 2.08E-02 | 937E.01 | 12 | 3.21E-03 | 3.07E-02 | -1.6 |
| 17047086 | MIR590 | 9.43E-01 | 9.99E-01 | 1.0 | 9.98E-01 | 9.4SE.01 | -1.0 | 4.64E-02 | 1.1OE-01 | -1.6 |
| 16792454 | MIR548Y | 1.44E-01 | 9.99E.01 | -1.1 | 8.37E-01 | 9.41E-01 | -1.0 | 3.25E-02 | 9.09E-02 | -1.5 |
| 16988869 | MIR4633 | 3.95E-01 | 9.99E.01 | 1.1 | 2.12E-02 | 9.37E.01 | 1.4 | 2.64E-02 | 8.19£-02 | 1.5 |
| 16913617 | MIR54802 | 3.35E-01 | 9.99E-01 | 1.3 | 8.50E-01 | 9.41E.01 | 1.0 | 2.25E-01 | 2.54E-01 | -1.5 |
Table 1: miRNA regulation in response to TGF-β, C3a and C5a for 24 h in normal primary human SAECs.
Discussion
Our results presented herein provide new insights on the hierarchical relationship between the complement cascade and TGF-β signaling. Our results suggest that TGF-β-induced lung fibrosis is arrested by blocking complement signaling. We also observed that TGF-β-induced local complement activation is suppressed by blocking complement signaling. Furthermore, TGF-β-induced loss of inhibitors of the two cascades, i.e. Smad7 (negative modulator of TGF-β) and DAF (early inhibitor of the complement cascade) is recovered. in vitro , we demonstrate that TGF-β-induced loss of DAF is prevented by RNAi-mediated silencing of the receptors to complement. Blockade of the TGF-β receptor kinase prevented the loss of C3a -mediated loss of DAF. Interestingly, both C3a and C5a share miRNA regulation in common with TGF-β of those common with epithelial proliferation, fibrosis and tumor biology. Cumulatively, our data suggest that while complement signaling may not be upstream of that of TGF-β, they share parallel mechanisms in mediating lung fibrosis.
It is well-established that genetic silencing or the pharmacologic blockade of the receptors for C3a or C5a results in suppression of the pro-inflammatory/pro-fibrotic molecule, TGF-β, in experimental models of lung fibrosis [4], sepsis [32], hepatic metastases of colon cancer [33] and immunoglobin A nephropathy [34].
Although each of these reports had investigated and reported the effects of blocking complement on TGF-β, none of these reports had interrogated the hierarchical relationship of complement and TGF-β. Based on our prior report [4], we hypothesized that since genetic silencing of the receptors for C3a or C5a suppresses TGF-β-related signaling and hence bleomycin-induced lung fibrosis, then genetic silencing of the receptors for C3a or C5a should not protect against TGF-β-induced lung fibrosis. However, we detected an arrest in lung fibrosis. Our data suggest that complement activation most likely enhances TGF-β signaling and shares signaling pathways and miRNA regulation related to lung fibrosis. Lam et al [21] had reported that deficiency of Wnt coreceptor, lrp5, resulted in a reduced production of bleomycininduced TGF-β in the lungs. Therefore, they had hypothesized that if lrp5 were upstream of TGF-β, then there should not be any protection in the lrp5-deficient mice in TGF-β-induced lung fibrosis and reported observations that supported their hypothesis. In our studies, we observed a combined recovery of both Smad7 and DAF-the inhibitors of TGF-β and the complement cascade, respectively. This would explain the reason underlying the protection against TGF-β-induced lung fibrosis. To the best of our knowledge, this is the first report to demonstrate the hierarchical relationship of a major innate immune pathwaythe complement cascade, when activated by the pro-fibrotic cytokine-TGF-β. Our studies suggest that these two cascades mediate fibrosis by functioning in parallel.
We had recently reported higher hemolytic activity in IPF patients [3]. Recent clinical reports indicate that targeting the complement cascade is gaining traction, e.g. C3 (compstatin/POT- 4 peptide) [35], C5 antibody (Soliris; Alexion Pharmaceuticals) [36], Factor D (TNX-234 antibody) [37], C5ar1 inhibitor, C1- INH (various manufacturers) in treating Paroxysmal Nocturnal Hemoglobinuria, atypical Hemolytic Uremic Syndrome, ARMD, gastrointestinal injury-related GVHD, C3 nephropathy, amyotrophic lateral sclerosis and other severe disorders. Our results in this report show that limiting complement activation may be a promising therapeutic strategy against TGF-β-induced lung fibrosis and is consistent with the report on the bleomycin injury model [4]. Therefore, the number of complement inhibitors previously used in clinical trials targeting other diseases can now be extended as a therapy for lung fibrosis.
Since the TGF-β/Smad2/3 and bone morphogenic proteins [4,7] /Smad1 signaling pathway has been implicated in clinical IPF [38] and in the murine bleomycin model of IPF [16], the effect of complement inhibition in Smad regulation was investigated. TGF-β1 signaling occurs via Type I and II receptormediated phosphorylation, whereby activated TGF-β1 receptor I phosphorylates Smad2 and Smad3 (receptor Smads, or R-Smads) at C-termini. R-Smads then complex with other molecules, translocate to the nucleus, and activate extracellular matrix gene transcription, enhancing fibrosis. R-Smads, particularly at the linker region, have also been shown to be phosphorylated by MAPK. R-Smad phosphorylation is antagonized by inhibitory Smad6 [39] and Smad7 [16] overexpression, which down-regulates TGF-β-induced activity and fibrosis. Smad6 and Smad7 are known antagonists of TGF-β signaling [40,41]. To our knowledge, this is the first report demonstrating the recovery of the inhibitory Smad7 via the suppression of complement activity. We next interrogated the inhibitor of the complement cascade-DAF. DAF is a glycosylphosphatidylinositol (GPI)-anchored membrane protein that restricts the synthesis of C3 and C5 convertases, and minimizes deposition of C3 and generation of C3a , C5a and C5b-9 [42]. It has a serine/threonine-enriched spacer domain and four complement control protein (CCP) repeats, which are tethered to the cell membrane by the GPI anchor [42]. When DAF binds to C3, the CCP2/3 domains prevent C3 formation and accelerate the decay of the C3 convertases C3bBb (alternative pathway) and C4b2a (classical and mannose-binding lectin pathways) [43]. Our data indicates that release of DAF onto the airway lumen due to TGF-β-induced lung injury is prevented by silencing the expression of the complement receptors. We also demonstrate this effect in vitro . This is consistent with our previous findings using pharmacologic blockade of TGF-β-induced loss of DAF in vitro .
Finally, we also interrogated the effects of blocking the TGF-β type I receptor-ALK5 (Alkivin like 5). Interestingly, we observed that regardless of a longer (48 h-SAECs) or a shorter (6h-AECs) exposure of C3a , pharmacologic blockade of ALK5 prevented the loss of DAF. This indicates a possibility of an autocrine effect, wherein C3a may induce loss of DAF via TGF-β. While we have established in our prior report that SAECs synthesize TGF-β transcripts at 16-24 h in response to C3a , it is unlikely that TGF-β transcripts are made as early as 4-6 h in AECs. Overall, our data provide mechanistic insights underlying complement inhibition in arresting TGF-β-induced lung fibrosis.
Till date, the miRNA studies have focused on regulation due to TGF-β in the pathogenesis of lung fibrosis. To the best of our knowledge, our results are the first to interrogate the effect of C3a or C5a on miRNA regulation in normal primary human small airway epithelial cells. While the optimal temporal regulation of the miRNA warrants more stringent investigation, our results interestingly show that at 24 h, all three agonists upregulated miR- 494 which has been implicated in the upregulation of ATF6 [44] or activated transcription factor isoform 6, a known endoplasmic reticular (ER) stress-related protein. ER stress has been implicated in epithelial injury, specifically in the lungs of patients with IPF [3,45,46]. While TGF-β signaling has been implicated in the downregulation of mir-200c and this was also observed in the lungs of IPF patients [28], interestingly our studies in normal primary human SAECs detected C5a -mediated downregulation of this miRNA. However, we detected upregulation of other classic miRNAs consistent with previous findings that they are implicated in lung fibrosis such as miR-21 [14], miR-31HG [26] and miR-503 [47,48]. Interestingly polymorphism in miR-4302 upregulated by C3a was associated with greater survival in lung cancer [49]. Our studies indicate that the complement cascade shares some miRNA regulation in the pathogenesis of lung fibrosis.
Our study has some potential limitations. First, although we have shown that TGF-β-induced lung fibrosis activates complement, we have not shown the specific mechanisms inducing the release of membrane-bound DAF are unknown. Secondly, the miRNA regulations are from 5 different normal donor lungs and the temporal responses and the contextual functions are yet to be characterized. The above limitations will shape our prospective studies.
Conclusion
In conclusion, our results suggest that the therapeutic blockade of C3a and C5a from binding to their respective receptors, C3aR and C5ar, using siRNA, arrests the progression of TGF-β-induced lung fibrosis by limiting complement activation. Additionally, the expression of the inhibitors of the two cascades i.e., SMAD7 and DAF, are restored, which will thus limit further signaling due to these pro-fibrotic mediators. Finally, we present some evidence showing that the two cascades share overlapping miRNA regulations which are potentially implicated in the pathogenesis of lung fibrosis.
Acknowledgement
Authors’ contributions
Conception and design of the study (RV); Conduct of the experiments and data acquisition (HG, AJF, EAM, AV); Data interpretation and analyses, drafting the manuscript, and revising the manuscript critically for important intellectual content (RV).
Funding Support Sources
NIH-NHLBI-R01-HL109288 (RV)
Conflict of Interest
The authors declare no conflict of interest
References
- Vittal R, Fan L, Greenspan DS, Mickler EA, Gopalakrishnan B, et al. (2013) IL-17 induces type V collagen overexpression and EMT via TGF-beta-dependent pathways in obliterative bronchiolitis. Am J Physiol Lung Cell MolPhysiol 304: L401-L414.
- Gu H, Mickler EA, Cummings OW, Sandusky GE, Weber DJ,et al. (2014) Crosstalk between TGF-beta1 and complement activation augments epithelial injury in pulmonary fibrosis. FASEB J 28: 4223-4234.
- Cipolla E, Fisher AJ, Gu H, Mickler EA, Agarwal M, et al. (2017) IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J.
- Gu H, Fisher AJ, Mickler EA, Duerson F 3rd, Cummings OW, et al. (2016) Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis. FASEB J.
- Jansen HM, Schutte AJ, Elema JD, Giessen MV, Reig RP, et al. (1984) Local immune complexes and inflammatory response in patients with chronic interstitial pulmonary disorders associated with collagen vascular diseases. Am J ClinExp Immunol 56: 311-320.
- Dreisin RB, Schwarz MI, Theofilopoulos AN, Stanford RE (1978) Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med 298: 353-357.
- Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, et al. (2013) Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol 191: 4431-4439.
- Cravedi P, Leventhal J, Lakhani P, Ward SC, Donovan MJ, et al. (2013) Immune cell-derived C3a and C5a costimulate human T cell alloimmunity. Am J Transplant 13: 2530-2539.
- Drouin SM, Kildsgaard J, Haviland J, Zabner J, Jia HP, et al. (2001) Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J Immunol 166: 2025-2032.
- Onishi A, Sugiyama D, Kumagai S, Morinobu A (2013) Cancer incidence in systemic sclerosis: Meta-analysis of population-based cohort studies. Arthritis Rheum 65: 1913-1921.
- Cushing L, Kuang PP, Qian J, Shao F, Wu J, et al.(2011) MiR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell MolBiol 45: 287-294.
- Li S, Geng J, Xu X, Huang X, Leng D, et al. (2016) MiR-130b-3p modulates epithelial-mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PloS one 11: e0150418.
- Lino Cardenas CL, Henaoui IS, Courcot E, Roderburg C, Cauffiez C, et al. (2013) MiR-199a-5p is upregulated during fibrogenic response to tissue injury and mediates TGFbeta-induced lung fibroblast activation by targeting caveolin-1. PLoS Genet 9: e1003291.
- Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, et al. (2010) MiR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207: 1589-1597.
- Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, et al. (2010) Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J RespirCrit Care Med. 182: 220-229.
- Vittal R, Fisher A, Gu H, Mickler EA, Panitch A, et al. (2013) Peptide-mediated inhibition of mitogen-activated protein kinase-activated protein kinase-2 ameliorates bleomycin-induced pulmonary fibrosis. Am J Respir Cell MolBiol 49: 47-57.
- Vittal R, Mickler EA, Fisher AJ, Zhang C, Rothhaar K, et al. (2013) Type V collagen induced tolerance suppresses collagen deposition, TGF-beta and associated transcripts in pulmonary fibrosis. PloS one 8: e76451.
- Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, et al. (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury.Nat Med 15: 1077-1081.
- Tarantal AF, Chen H, Shi TT, Lu CH, Fang AB, et al. (2010) Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. EurRespir J 36: 907-914.
- Rodt T, Von Falck C, Dettmer S, Halter R, Maus R, et al. (2010) Micro-computed tomography of pulmonary fibrosis in mice induced by adenoviral gene transfer of biologically active transforming growth factor-beta1. Respir Res11: 181.
- Lam AP, Herazo-Maya JD, Sennello JA, Flozak AS, Russell S, et al. (2014) Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am J RespirCrit Care Med 190: 185-195.
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, et al. (2004) Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 173: 2099-2108.
- De Boer JD, Van't Veer C, Stroo I, Van Der Meer AJ, De Vos AF, et al. (2013) Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun 20: 618-625.
- Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, et al. (2013) Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol 191: 4431-4439.
- Jiang X, Nguyen TT, Tian W, Sung YK, Yuan K, et al. (2015) Cyclosporine does not prevent microvascular loss in transplantation but can synergize with a neutrophil elastase inhibitor, elafin, to maintain graft perfusion during acute rejection. Am J Transplant 15: 1768-1781.
- Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, et al. (2012) Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Bio 47: 879-889.
- Yang S, Cui H, Xie N, Icyuz M, Banerjee S, et al. (2013) MiR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J 27: 2382-2391.
- Yang S, Banerjee S, De Freitas A, Sanders YY, Ding Q, et al. (2012) Participation of miR-200 in pulmonary fibrosis. Am J Pathol 180: 484-493.
- Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, et al. (2013) Epigenetic regulation of miR-17~92 contributes to the pathogenesis of pulmonary fibrosis. Am J RespirCrit Care Med 187: 397-405.
- Benoit ME, Tenner AJ (2011) Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci 31: 3459-3469.
- Eadon MT, Jacob A, Cunningham PN, Quigg RJ, Garcia JG, et al. (2014) Transcriptional profiling reveals that C5a alters microRNA in brain endothelial cells. Immunology 143: 363-373.
- Silasi-Mansat R, Zhu H, Georgescu C, Popescu N, Keshari RS, et al. (2015) Complement inhibition decreases early fibrogenic events in the lung of septic baboons. J Cell Mol Med 19: 2549-2563.
- Piao C, Cai L, Qiu S, Jia L, Song W, et al. (2015) Complement 5a enhances hepatic metastases of colon cancer via monocyte chemoattractant protein-1-mediated inflammatory cell Iifiltration. J BiolChem 290: 10667-10676.
- Zhang Y, Yan X, Zhao T, Xu Q, Peng Q, et al. (2017) Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin a nephropathy in mice. ClinExp Immunol 189: 60-70.
- Ricklin D, Lambris JD (2008) Compstatin: A complement inhibitor on its way to clinical application. Adv Exp Med Biol 632: 273-292.
- Mevorach D, Reiner I, Grau A, Ilan U, Berkun Y, et al. (2016) Therapy with eculizumab for patients with CD59 p.Cys89Tyr mutation. Ann Neurol 80: 708-717.
- Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, et al. (2017) Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med 9.
- Fernandez IE, Eickelberg O (2012) The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am ThoracSoc 9: 111-116.
- Murray LA, Hackett TL, Warner SM, Shaheen F, Argentieri RL, et al. (2008) BMP-7 does not protect against bleomycin-induced lung or skin fibrosis. PloS one 3: e4039.
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, et al. (1997) The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling.Cell 89: 1165-1173.
- Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, et al. (1999) Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 104: 5-11.
- Lublin DM, Atkinson JP (1989) Decay-accelerating factor: Biochemistry, molecular biology, and function. Annu Rev Immunol 7: 35-58.
- Kuttner-Kondo L, Hourcade DE, Anderson VE, Muqim N, Mitchell L, et al. (2007) Structure-based mapping of DAF active site residues that accelerate the decay of C3 convertases. J BiolChem 282: 18552-18562.
- Oglesby IK, Agrawal R, Mall MA, McElvaney NG, Greene CM (2015) MiRNA-221 is elevated in cystic fibrosis airway epithelial cells and regulates expression of ATF6. Mol Cell Pediatr 2: 1.
- Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, et al. (2008) Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell MolPhysiol 294: L1119-L1126.
- Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, et al. (2008) Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J RespirCrit Care Med 178: 838-846.
- Markopoulos GS, Roupakia E, Tokamani M, Vartholomatos G, Tzavaras T, et al. (2017) Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. ExpGerontol 96: 110-122.
- Zhu H, Li Y, Qu S, Luo H, Zhou Y, et al. (2012) MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol 32: 514-522.
- Zhao Y, Wei Q, Hu L, Chen F, Hu Z, et al. (2014) Polymorphisms in MicroRNAs are associated with survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 23: 2503-2511.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences