Calreticulin and JAK2V617F Mutations in Essential Thrombocythemia and Their Potential Role in Diagnosis and Prognosis
Najmaldin Saki
Ahmad Ahmadzadeh1, Reza Shirzad1, Neda Golchin1, Saeid Shahrabi2, Mohammad Shahjahani1,Mohammad Seghatoleslami1, Najmaldin Saki2*
1Health research institute, Research Center of Thalassemia & Hemoglobinopathy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2Department of biochemistry and hematology, Faculty of medicine, Semnan University of medical sciences, Semnan, Iran
- *Corresponding Author:
- Najmaldin Saki
Health Research Center, Research Institute of Thalassemia and Hemoglobinopathy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Tel: +98.6113738317
Fax: +98.611 3738330
E-mail: najmaldinsaki@gmail.com
Received date: September 28, 2015; Accepted date: December 11, 2015; Published date: December 16, 2015
Abstract
Mutation in calreticulin (CALR) has been recently detected in essential thrombocythemia (ET) patients lacking JAK2V617F mutation. The absence of JAK2V617F mutation in 50% of patients with ET has indicated the importance of new markers for disease diagnosis, including CALR. Considering the detection of CALR in these patients, in this paper we study the structure and function of CALR protein and JAK2V617F as well as their diagnostic and prognostic role in ET patients.
Keywords
Essential thrombocythemia; Calreticulin; JAK2V617F
Abbreviations
MPNS: Myeloproliferative Neoplasms; ET: Essential Thrombocythemia; CALR: Calreticulin; JAK2: Janus kinase2; BM: Bone Marrow; TPO: Thrombopoietin; EPO: Erythropoietin; PMF: Primary Myelofibrosis; MRD: Minimal Residual Disease; MPL: Myeloproliferative Leukemia
Introduction
ET was first recognized in 1934 by the name of hemorrhagic thrombocythemia. ET is characterized by an unusual thrombocytosis and risk of thrombotic and hemorrhagic disorders. The annual incidence of ET is approximately 1-2.5 per 100,000 population [1]. The incidence of ET is higher in those 50-70 years old, and is most often incidentally detected during a high platelet count. A number of patients have thrombotic and hemorrhagic disorders and a small number of them show disease transformation towards myelofibrosis, myelodysplasia or AML [2]. ET patients rarely show two simultaneous mutations. For example, mutation in JAK2V617F or JAK2 exon 12 rarely coincides with mutation in exon 10 MPL gene in ET [3-6]. Hence, since the definite diagnosis of ET is not feasible in patients without mutation in JAK2V617F, new biomarkers are required to identify such patients, including the newly discovered CALR protein. Remarkably, mutation in CALR has been detected in 29-80% of ET patients lacking mutation in JAK2V617F. Moreover, ET patients with mutation in CALR show better prognosis than patients with JAK2V617F mutation lacking CALR mutation [7-11]. Mutation in CALR and JAK2V617F simultaneously occur in approximately 80% of ET patients. ET patients lacking mutation in both CALR and JAK2V617F can lack any mutation or show mutation in TP53, DNMT3A, TET2, MPL, NFE2, CUX1 and del 7q [12]. As a result, CALR mutation assay in ET patients, especially those lacking mutation in JAK2V617F, can be helpful in disease prognosis and diagnosis. Given the role and function of CALR and JAK2V617F in ET patients, in this review we have attempted to study the structure and function of CALR and JAK2V617F, their mutation and interaction in ET in order to better understand disease prognosis and diagnosis. In addition, we have attempted to suggest novel CALR-based therapeutic strategies according to different functions of CALR to help control the disease in ET patients, which requires further investigations.
Materials and Methods
Literature search and search strategy
Given the recent discovery of CALR mutation in patients with ET harboring JAK2V617 mutation, Pubmed and Medline search engines were searched from 1995 to 2014 and the papers were selected with respect to inclusion and exclusion criteria. The keywords selected for search from MeSH database include Essential thrombocythemia; JAK2V617F and calreticulin. The diagnosis of ET is based on criteria released in 2001 or 2008 by WHO and British Committee for Standards in Hematology (BCSH), respectively [13- 15]. In the majority of studies, ET patients were selected from institutional databases based on availability of archived bone marrow or peripheral blood DNA for mutation screening, Sanger sequencing or allele-specific PCR (AS-PCR) to detect mutations, including CALR and JAK2V617F.
Inclusion criteria
After searching the databases, studies regarding JAK2V617F, CALR and ET were collected. Then, since evaluation of simultaneous prevalence of JAK2V617F and CALR mutations in ET patients was the main inclusion criteria, the papers assessing both CALR and JAK2V617F mutations in ET patients entered into our study. In addition, studies evaluating the incidence of thrombosis and other laboratory findings such as Hb, Plt and WBC counts as well as detecting the above-mentioned mutations were included. Finally, the data were collected and analyzed (Figure 1).
Exclusion criteria
Studies dealing with the prevalence of JAK2V617F and CALR mutations in MPNs other than ET were excluded. Afterwards, studies that only measured the prevalence of CALR but not JAK2V617F and vice versa were also excluded. A number of papers had evaluated JAK2V617F and CALR mutations but no other laboratory findings like thrombosis, Hb, Plt and WBC count and were also excluded.
JAK2 as a main biomarker in ET diagnosis and prognosis
ET patients are divided to two groups: those bearing JAK2V617F mutation and those lacking it [16-22]. Patients with JAK2V617F mutation commonly have hemoglobin levels lower than 16 g/dL, WBC count lower than 9.0 × 109/L and BM with higher cellularity [7-11]. These values are different in ET patients bearing CALR mutation, and lower Hb and WBC values with a higher platelet count have been reported in them [7-11]. A higher risk of thrombosis and vascular abnormalities has also been reported in ET patients bearing JAK2V617F mutation [16-22]. Therefore, this group of ET patients shows a poorer prognosis than patients lacking this mutation (Tables 1 and 2).
| Positive JAK2V617F | NegativeJAK2V617F | Reference | |
|---|---|---|---|
| Patients Number | 77 | 34 | [17] |
| 76 | 103 | [21] | |
| 73 | 77 | [16] | |
| 380 | 366 | [20] | |
| 61 | 47 | [22] | |
| 60 | 46 | [19] | |
| 79 | 94 | [18] | |
| Thrombosis (%) | 35 (46.7%) | 10 (29.4%) | [17] |
| 34 (33%) | 13 (17%) | [21] | |
| 17 (23.3%) | 15 (19.5%) | [16] | |
| 53 (14.0%) | 24 (6.6%) | [20] | |
| 21 (34.4%) | 14 (29.8%) | [22] | |
| 20 (33.3%) | 4 (8.6%) | [19] | |
| WBC (×109L) (median, S.D.) | 10.9 ± 4.2) | 10.3 ± 3.4 | [17] |
| 9.9 ± 0. | 8.1 ± 0.3 | [21] | |
| 10.7(5.0-24.1) | 9.1(4.0-29.8) | [16] | |
| 11.1 (4.0-29.6) | 8.9 (4.0-24.8) | [20] | |
| 10.7 (5.7-26.1) | 8.9 (3.7-29.4) | [22] | |
| 9.2 ± 2.8 | 8.1 ± 2.0 | [19] | |
| 11.69±5.47 (5.8-43.8) | 11.48 ± 7.82 (4.3-49) | [18] | |
| Hb (g/dL) (median, S.D.) | 13.8 ± 1.7 | 13.1 ± 1.8 | [17] |
| 14.4 ± 0.14 | 13.5 ± 0.14 | [21] | |
| 14.6 (10.9-17.6) | 14.0(9.0-16.4) | [16] | |
| 14.5 (53-16.4) | 13.3 (5.1-16.4) | [20] | |
| 13.6 (10.0-17.3) | 12.8 (7.3-17.5) | [22] | |
| 14.40±1.64 | 13.05±2.3 | [18] | |
| PLT (×109L−1) (median, S.D.) | 900 ± 347 | 963 ± 316 | [17] |
| 767 ± 26 | 858 ± 35 | [21] | |
| 1000 (509-3000 | 1000 (454-3460) | [16] | |
| 808 (458-3958) | 969 (460-3635 | [20] | |
| 942 (474-2,271) | 954 (608-2,333) | [22] | |
| 650 ± 145 | 766 ± 195 | [19] | |
| 867.9±332.58 | 874.89±370.26 | [18] | |
| Prognosis of ET | poor | Controversial | [16-22] |
| Diagnosis of ET | confirm | Controversial | [16-22] |
Table 1: Clinical findings of ET patients and their application in diagnosis and prognosis.
| Positive JAK2V617F | Positive CALR | Reference | |
|---|---|---|---|
| Patients Number | 240 | 99 | [10] |
| 466 | 176 | [8] | |
| 227 | 114 | [7] | |
| 369 | 89 | [9] | |
| 154 | 96 | [11] | |
| Thrombosis (%) | 72 (30.0) | 13 (13.1) | [10] |
| 33 (7.1%) | 5 (2.8%) | [8] | |
| 86 (38%) | 28 (25%) | [7] | |
| 111 (30.1) | 12 (13.5) | [9] | |
| 27 (18%) | 6 (7%) | [11] | |
| WBC (×109L) (median, S.D.) | 11.1 (4.0-27.5) | 8.6 (4.2-17.1) | [10] |
| 9.0 (4.0-28.0) | 8.0 (4.0-17.9) | [8] | |
| 9.8 (0.6-53.4) | 8.3 (3.3-32.6) | [7] | |
| 8.9 (4.2-35.0) | 8.1 (3.5-26.0) | [9] | |
| 10 (8-13) | 9 (8-12) | [11] | |
| Hb (gr/dL) (median, S.D.) | 14.4 (66-165) | 12.8 (85-165) | [10] |
| 14.4 (10-17.7) | 13.8 (11.3-17.6) | [8] | |
| 14.1 (9.8-17.9) | 13.3 (6.9-16.4) | [7] | |
| 14.5 (102-173) | 13.8 (106-173) | [9] | |
| 14.7 (134-156) | 13.1 (120-145) | [11] | |
| PLT (×109L) (median, S.D.) | 734 (461-2421) | 1060 (590-3340) | [10] |
| 700 (456-2148) | 883 (500-3000) | [8] | |
| 862 (453-3000) | 1100(454-3460) | [7] | |
| 726 (455-1881) | 866 (504-2348) | [9] | |
| 778 (651-992) | 981 (767-1389) | [11] | |
| Prognosis of ET | poor | good | [7-11] |
| Diagnosis of ET | confirm | confirm | [7-11] |
Table 2 Clinical findings of ET patients harboring JAK2V617F and CALR mutation, and their use in disease diagnosis and prognosis.
JAK2V617F mutation in exon 14 of chromosome 9 is a common mutation in identification of MPNs, including ET, which plays an important role in signaling of cytokines (including TPO) affecting hematopoietic cells [23]. This pathway is involved in cellular mechanisms, including proliferation, survival, hematopoiesis and immune function [24]. Each JAK2V617F protein includes an active tyrosine kinase domain of JAK2V617F homology1 (JH1), an inactive catalytic domain of JH2, Src homology domain 2 (SH2) and a 4-point-1erzin radixin moesin (FERM) domain in the amine terminal (Figure 2A) [23,24]. A point mutation in exon 14 of JAK2V617F gene results in substitution of codon 617 (valine) with phenylalanine in the JH2 domain, which causes inactivation of its inhibitory effect on JAK2V617F, through which tyrosine kinase activity in JAK2V617F is induced in the absence of TPO and EPO ligands. The expression of JAK2V617F mutation causes sustained activity of JAK2V617F signaling pathway as well as other signaling pathways, resulting in increased sensitivity of megakaryocyte and leukocyte precursors to growth factors, causing their cytokine independent proliferation [25]. This mutation triggers the cytokine-independent activation of JAK/STAT, PI3, AKT and MEK/ ERK pathways (Figure 2B), all of which are involved in signaling of hematopoietic factors such as TPO, EPO and Granulocyte- Colony Stimulating Factor [26]. JAK2V617F signal transduction pathway plays an important role in cell signaling, and mutations in it can be of clinical importance [27]. Since JAK2V617F pathway causes ineffective hematopoiesis and megakaryopoiesis as well as splenomegaly in patients, the patients bearing this mutation have a higher incidence of thrombotic disorders with higher leukocyte count and hemoglobin concentration than in patients lacking mutation. JAK2V617F inhibitors like Ruxolitinib are involved in reducing ineffective hematopoiesis, megakaryopoiesis and splenomegaly in ET patients [25]. Mutation in JAK2V617F is present in 80-90% of patients with Polycythemia Vera (PV), 50-58% of patients with ET, 50-65% of patients with idiopathic PMF and 20% of non-classified MPN patients [28-31]. It is also observed in a small group of myelodysplastic syndromes, acute myelomonocytic leukemia, acute myeloid leukemia (AML), chronic myelogenous leukemia (CML) and other MPNs such as Philadelphia negative myeloid leukemia [32]. Although JAK2V617F mutation was first reported to be negative in CML with Philadelphia positive chromosome, subsequent studies have confirmed the presence of mutant JAK2V617F in CML [33-36]. In presence of this mutation, overactive JAK2V617F causes increased production of megakaryocyte, erythroid and granulocytic series with the expansion of erythron associated with splenomegaly [37,38]. ET patients bearing JAK2V617F mutation show a stronger prothrombotic status due to significant increase in the expression of tissue factor, fibrinogen and a high tendency to form leukocyteplatelet aggregates. Therefore, this mutation may be one reason for the increased thrombotic disorders and poorer prognosis in these patients, whereas there are fewer thrombotic disorders and better prognosis in patients lacking this mutation [16-22]. Moreover, JAK2V617F and MPL mutations have been reported in 50-60% and 5-10% of ET patients, respectively. However, 30-45% of ET patients lack these mutations, which has caused contradictions and difficulties in diagnosis of ET by physicians [31]. Therefore, finding new markers contributing to definitive diagnosis and prognosis of TE patients is inevitable.
Figure 2: A) Location of JAK2V617F mutation and other exon 14 mutations (from SH2 domain) in JAK2. Janus kinase (JAK2) mutations could exist as point mutations, duplications and insertions in essential thrombocythemia (ET) and other MPNs. Location of these mutations is the regulatory N terminal of pseudokinase, namely SH2 (Src-homology domain). JAK2V617F mutation is the most frequent mutation in these patients. B) Schematic figure of JAK2V617F mutation and TPO signaling. JAK2V617F mutation activates the transcription factors involved in myeloid and megakaryocytic proliferation, and can be assumed as the original cause of MPNS such as ET. Activated JAK2 kinase phosphorylates the tyrosine of STAT, which is dimerized and enters the nucleus, binding the regulatory sequence of target gene to regulate its transcription and increase the proliferation of myeloid and megakaryocytic progenitors. On the other hand, JAK2 phosphorylates Ras/MEK/ERK molecules that result in cell proliferation. Therefore, JAK2V617F mutation by STAT, Ras/ MEK/ERK and PI3K/Akt pathways results in proliferation of myeloid and megakaryocytic progenitors in the absence of TPO. JAK2 inhibitors (e.g. Ruxolitinib) inhibit both mutated and non-mutated JAK2 and do not discriminate between them.
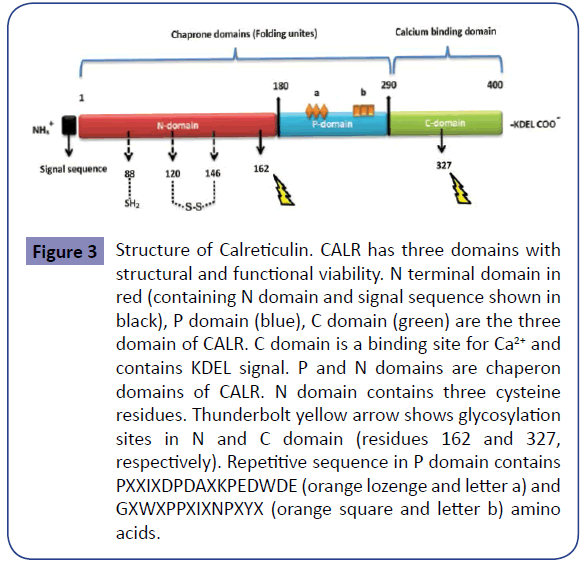
Figure 3: Structure of Calreticulin. CALR has three domains with structural and functional viability. N terminal domain in red (containing N domain and signal sequence shown in black), P domain (blue), C domain (green) are the three domain of CALR. C domain is a binding site for Ca2+ and contains KDEL signal. P and N domains are chaperon domains of CALR. N domain contains three cysteine residues. Thunderbolt yellow arrow shows glycosylation sites in N and C domain (residues 162 and 327, respectively). Repetitive sequence in P domain contains PXXIXDPDAXKPEDWDE (orange lozenge and letter a) and GXWXPPXIXNPXYX (orange square and letter b) amino acids.
Minimal residual disease and JAK2
Since the detection of JAK2V617F mutation is important in the diagnosis of MPNs, sensitive and specific detection methods of JAK2V617F mutation can be helpful for monitoring of the disease. New diagnostic techniques such as sequencing not only contribute to better diagnosis and treatment of ET, but may be helpful in MRD detection and monitoring the response to have shown that the detectable level of JAK2V617F mutation is <l% in some cases, which is lower than the level detected in a number of standard diagnostic techniques such as pyrosequencing [40]. Therefore, new techniques like qPCR can be suitable to detect the point mutations, including JAK2V617F, and can contribute to molecular detection of MRD and selection of therapeutic methods [32,41].
Significance of CALR as a new biomarker in ET
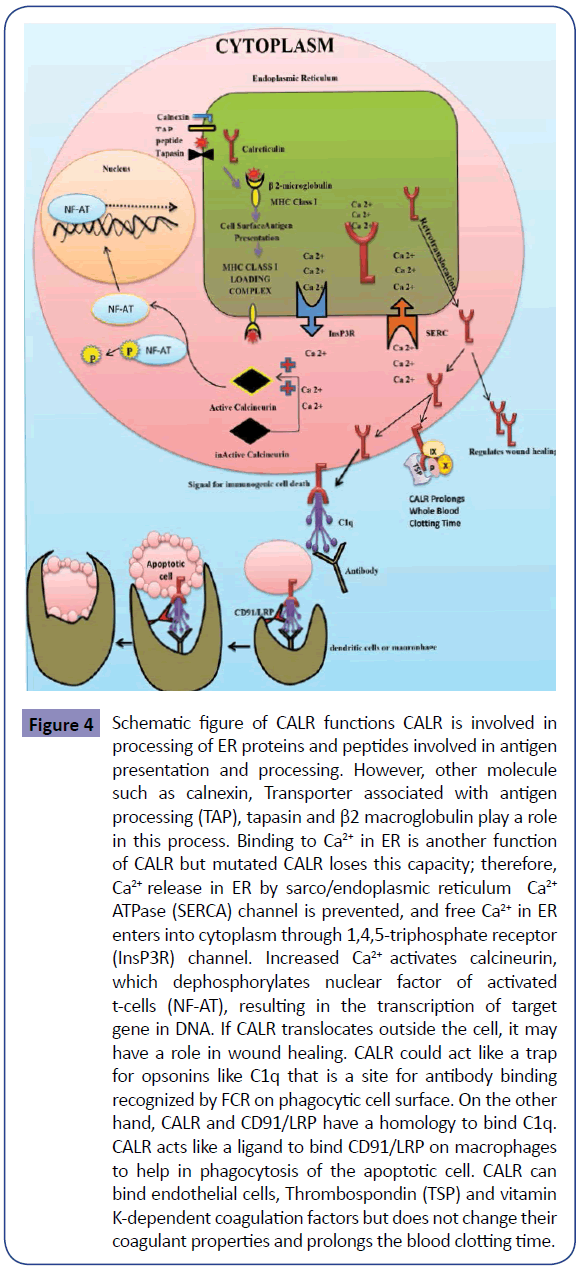
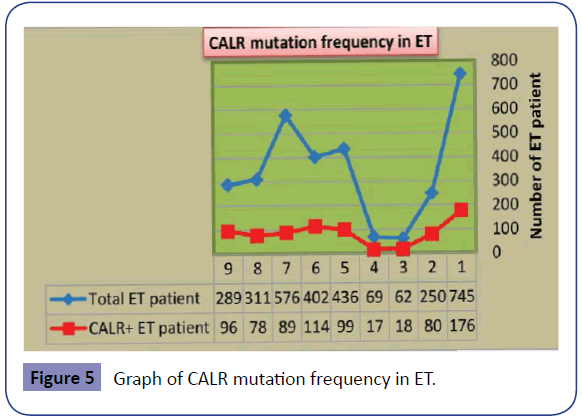
As noted above, JAK2V617F mutation is the most common mutation in ET patients [16-22], but it is not present in all ET patients. This has caused problems in definitive molecular diagnosis of ET patients lacking JAK2V617F mutation. This led researchers to try for discovery of new markers for the diagnosis of ET patients without JAK2V617F mutation. CALR is a newly discovered mutation and is the second most common mutated gene in ET patients [7-9,41]. As mentioned above, although the diagnosis of ET patients is confirmed by JAK2V617F mutation, the presence of this mutation is an indication of poorer prognosis relative to patients without JAK2V617F mutation (Table 1) [16-22]. In contrast, the diagnosis of ET patients bearing CALR mutation is not only confirmed but indicates a better prognosis than patients with JAK2V617F mutation (Table 2) [7-11]. The data collected indicate the importance of CALR mutation in prognosis of ET patients. CALR is a 46- kDa calcium binding protein within the lumen of endoplasmic reticulum, but it is also found in the nucleus, cell membrane and extracellular matrix. CALR is located in 19p13.2 locus, which contains nine exons in the size range of 4.2 Kb (Figure 3) [42]. CALR is involved in calcium homeostasis, transport of misfolded proteins, T-cell binding, immune response to cancer, phagocytosis, inhibition of apoptosis and activation of transcription factors such as NF-AT (Figure 4) [43]. Unusual down regulation of CALR has been reported in a variety of cancers, such as breast cancer, and is associated with reduced apoptosis of cancer cells [35,36]. Mutation in CALR and the absence of C-terminal domain results in loss of Ca2+ binding capacity [44]. The cells with low levels of CALR have decreased Ca2+ levels in ER and increased activity of calmodulin-Ca2+/calmodulin-dependent protein kinase II pathway [44,45]. Increased extracellular calcium due to deficiency or mutation in CALR can influence the development process of cells by preventing or activating the transcription factors. As a result, CALR is implicated in growth of cells such as lymphocytes and adipocytes [35,45]. Therefore, further research on CALR and its effect upon development of platelets and decreased growth of leukocytes in ET can be helpful. Thirty-six CALR mutations have been reported in PMF and ET. Type I deletion in exon 9 (52-bp deletion; c.1092_1143del) and type II insertion in exon 9 (5- bp insertion; c.1154_1155insTTGTC) are the most frequent mutations. The frequency of CALR mutation in ET and PMF has been reported to be higher than other MPNs, while 100% of PV patients have been reported negative for CALR mutation, and only one case of CML has been reported positive for CALR mutation [31,41]. The frequency of CALR mutation in patients with ET (regardless of positive or negative mutation of JAK2V617F) is estimated to be 15-30%. The frequency of CALR mutation in patients with ET is shown in Table 3 and Figure 5. Moreover, recent studies on 10 cases of familial MPN with a history of myeloproliferative disease showed that 2 out of 10 ET patients had a mutation in CALR, which indicated that the mutation is not restricted to sporadic MPNs and can also occur in familial cases [46]. Comparing Tables 1 and 2 shows that platelet count is higher in ET patients with CALR mutation than those bearing JAK2V617F mutation. WBC and hemoglobin levels as well as thrombosis occurrence are higher in ET patients bearing JAK2V617F mutation than patients with CALR mutation (Figure 6). These results and mutation diagrams along with laboratory data of patients from diagnosis through treatment can contribute to diagnosis, monitoring and prognosis of patients as well as MRD detection, offering a novel method to help patients, which requires further investigations.
Figure 4: Schematic figure of CALR functions CALR is involved in processing of ER proteins and peptides involved in antigen presentation and processing. However, other molecule such as calnexin, Transporter associated with antigen processing (TAP), tapasin and β2 macroglobulin play a role in this process. Binding to Ca2+ in ER is another function of CALR but mutated CALR loses this capacity; therefore, Ca2+ release in ER by sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) channel is prevented, and free Ca2+ in ER enters into cytoplasm through 1,4,5-triphosphate receptor (InsP3R) channel. Increased Ca2+ activates calcineurin, which dephosphorylates nuclear factor of activated t-cells (NF-AT), resulting in the transcription of target gene in DNA. If CALR translocates outside the cell, it may have a role in wound healing. CALR could act like a trap for opsonins like C1q that is a site for antibody binding recognized by FCR on phagocytic cell surface. On the other hand, CALR and CD91/LRP have a homology to bind C1q. CALR acts like a ligand to bind CD91/LRP on macrophages to help in phagocytosis of the apoptotic cell. CALR can bind endothelial cells, Thrombospondin (TSP) and vitamin K-dependent coagulation factors but does not change their coagulant properties and prolongs the blood clotting time.
Figure 6: Graph of laboratory findings in JAK2 positive/negative and CALR positive mutation in ET. The left graphs show the TBR, Hb, Plt and WBC in JAK2 positive/negative cases. Right graphs show the TBR, Hb, Plt and WBC in CALR positive/negative cases. These data could help in diagnosis, prognosis and determination of MRD in ET in correlation with qPCR data from diagnosis to CR. Abbreviations: Minimal residual disease (MRD), Complete remission (CR), Thrombosis (TBR), Hemoglobin (Hb), Platelet (Plt), White blood cell (WBC). Quantitative polymerase chain reaction (qPCR).
Coagulation disorders in ET and prognostic role of CALR
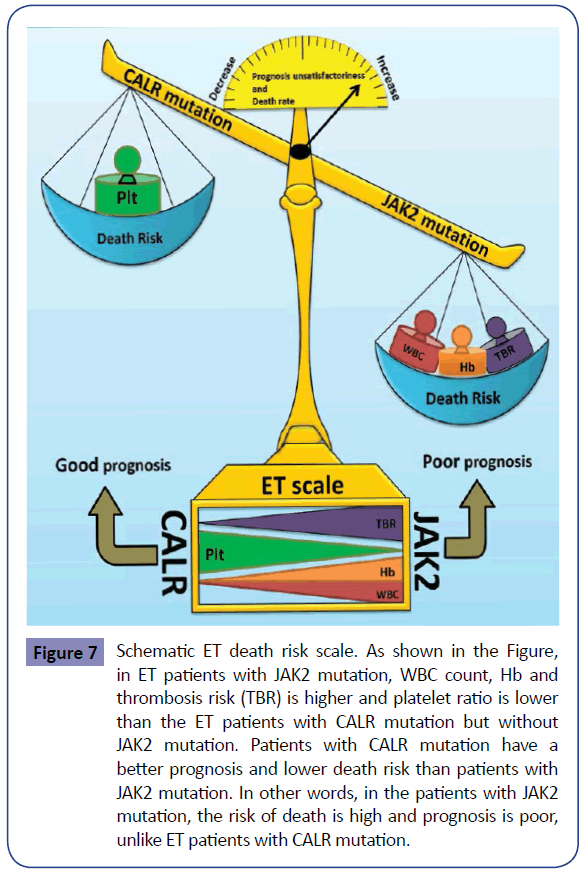
Most ET patients are asymptomatic and are fortuitously detected during CBC. A strong association has been found between JAK2V617F mutation and thrombotic disorders in ET [21], which are among ET manifestations. The nature of arterial and venous thrombosis is different in these patients. Arterial thrombosis mainly includes platelets (white clot) but venous thrombosis mostly consists of fibrin and red blood cells (red clot); however, several similarities between these two trends of thrombosis have been recently noted [47]. Studies have shown that leukocyte count is a better index for predicting thrombosis compared with platelet count, RBC and hematocrit [48]. In these patients, prothrombotic circulating endothelial cells have been reported, the majority of which bear the JAK2V617F mutation [49]. Cardiovascular disorders and thrombosis are more frequent in patients with mutant JAK2V617F compared to patients without the mutation [50,51]. Other symptoms of ET patients include deep vein thrombosis and pulmonary embolism [52-54]. ET patients bearing CALR mutation show lower hemoglobin and WBC than ET patients with JAK2V617F mutation, whereas the platelet count has been reported to be higher in ET patients with CALR mutation compared with those with JAK2V617F mutation (Figure 7). Studies indicated that ET patients with CALR mutation have a better thrombosis-free survival than patients with JAK2V617F mutation [55]. For example, among 300 Mayo Clinic ET patients, incidence of JAK2V617F and CALR mutations was reported 53% and 32%, respectively. In this study, ET patients with CALR mutation displayed better thrombosis-free survival compared to patients with JAK2V617F mutation [55]. The reduction in thromboembolic disorders may be due to decreased hemoglobin concentration and leukocyte count in ET patients with CALR mutation compared with those bearing JAK2V617F mutation [31,56,57]. CALR has been recognized as a calcium-binding protein since a decade ago. CALR binds the endothelial cells and vitamin K dependent coagulation factors (IX, X, VII and II) but causes no change in their coagulation activity (Figure 4). Moreover, CALR can interact with factor VIII and this interaction appears to be required for factor VIII secretion [58]. In animal models of thrombosis induced by electricity, Kuwabara et al found that intravascular injection of CALR helps prevent blood vessel occlusion [59]. Furthermore, CALR promotes angiogenesis via activating nitric oxide signaling pathway [60]. This activity is related to C-terminal domain of CALR, which is also found in platelets and may be associated with integrins [59]. The study of CALR as an anti-coagulant has shown that human and rabbit CALR dramatically prolong the whole blood clotting time. A similar increase was observed in clotting time of platelet-rich plasma but platelet poor plasma showed no difference in clotting time up to 300 seconds in the presence or absence of CALR. Therefore, CALR is likely to have anti-coagulation effect in association with platelet glycoproteins [59,61]. CALR acts as an anti-thrombotic agent [62,63]. As CALR can increase the clotting time, mutation in it causes a better prognosis in patients. Therefore, the possibility of a higher tendency of this molecule for vitamin K dependent coagulation factors and the resulting reduction in thrombotic disorders in ET patients could be one reason for the better prognosis in patients with this mutation, which requires further investigations. Several drugs like aspirin, hydroxyurea (HU), Anagrelide and JAK2 inhibitors are used to treat and control ET. Aspirin decreases both venous and arterial thrombosis [64,65]. Studies have shown that in patients with JAK2V617F mutation with risk factors (high-risk ET), prescription of aspirin can affect the incidence of venous thrombosis [66,67]. Studies have indicated that aspirin use prevents venous thrombosis in lowrisk ET patients bearing JAK2V617F mutation [66]. However, in ET patients lacking JAK2V617F mutation and bearing JAK2V617F mutation without risk factors (low-risk ET), cardiovascular risk factors and thrombotic disorders can be reduced by aspirin prescription [66]. HU halts DNA synthesis and decreases the platelet production and thrombosis in high-risk ET patients [68]. Anagrelide therapy in these patients causes decreased production of platelets [33,69]. JAK2V617F inhibitors such as INC18424 (Ruxolitinib) suppress the signal induced by JAK2V617F and decrease the platelet count [70]. The combination of old techniques with new methods can help improve the ET patients. The study of new molecules, their function and inactivation can present novel treatment approaches for control of ET. Hence, according to the anti-coagulant effects of CALR, further studies on this molecule and understanding its mode of action can be a future treatment approach for ET.
Figure 7: Schematic ET death risk scale. As shown in the Figure, in ET patients with JAK2 mutation, WBC count, Hb and thrombosis risk (TBR) is higher and platelet ratio is lower than the ET patients with CALR mutation but without JAK2 mutation. Patients with CALR mutation have a better prognosis and lower death risk than patients with JAK2 mutation. In other words, in the patients with JAK2 mutation, the risk of death is high and prognosis is poor, unlike ET patients with CALR mutation.
Simultaneous assessment of CALR and JAK2 in ET diagnosis and prognosis
ET patients are grouped based on risk factors, and previous history of thrombosis and age over 60 years are the most important risk factors. Increased leukocyte count at diagnosis is a predictive factor for thrombosis in ET and PV patients. ET patients with JAK2V617F mutation show a higher incidence of venous and arterial thrombosis compared with patients without this mutation, and this is also true in some patients with MPL mutation [51]. Increased BM reticulin fibrosis is a predictive factor for thrombotic and hemorrhagic disorders [33]. The study of BM and peripheral blood, application of WHO and BCSH criteria as well as JAK2V617F and MPL mutations are diagnostic approaches for ET. Hence, the discovery of new markers can help diagnose these patients. Since not all MPN patients bear the JAK2V617F mutation, detection of new mutations with a higher frequency (including CALR) can be helpful to better identify these patients. As previously mentioned (Figure 7), patients bearing CALR mutations show better survival and prognosis than ET patients lacking this mutation [55], which can help in the treatment and monitoring of these patients (Figure 8).
Figure 8: Diagnosis and prognosis algorithm of ET with JAK2 negative mutation by CALR. Distinction between MPN and CML is possible through the existence of Philadelphia (Ph) chromosome, which is the hallmark of CML. If Ph chromosome is absent, JAK2 mutation should be assessed in these patients. JAK2 mutation is present in most Ph negative MPN diseases such as ET, Primary myelofibrosis (PMF) and PV. In recent studies, CALR mutation has been assessed in Ph and JAK2 negative cases of MPN disorders. CALR mutation is completely negative and 60-80% positive in Ph and JAK2 negative PV and ET patients, respectively, and could be helpful for diagnosis of these patients.
Conclusion and future perspective
Given that the classification of hematopoietic and lymphatic tumors is undergoing developments by WHO revision, new findings and mutations like CALR and their interaction with other mutations such as JAK2V617F can help in classification, diagnosis, treatment monitoring and choice of clinical ET tests in other MPNs such as IMP and PV. CALR has been implicated in coagulation through binding platelets and their glycoproteins as well as vitamin K dependent coagulation factors. ET patients with increased platelet count and mutation in CALR show better prognosis and survival with a lower frequency of thrombotic disorders relative to those lacking this mutation, which is another indication of the association between CALR and coagulation. Therefore, since CALR binds to vitamin K dependent coagulation factors and increases the whole clotting time [59,61], we suggest the treatment or alleviation of symptoms in patients with thrombotic disorders via inhibition of CALR. Furthermore, the use of antibodies against CALR may be helpful for hemorrhagic complications in other disorders due to the role of CALR in increasing the clotting time. Mutated CALR can indirectly activate calcineurin via increased calcium levels. Mutated CALR has a lower capacity of Ca2+ binding, thus Ca2+ enters the cytoplasm from ER. This causes calcineurin activation, which causes transcription of NF-AT and affects the development process of the cells [36,43,44]. Considering low WBC count in ET patients bearing CALR mutation but high counts in those with JAK2V617F mutation, CALR may be implicated in development, growth and apoptosis of lymphocytes, adipocytes and cancer cells. Further studies on the role of CALR in the development process of WBC can be helpful in better recognition of ET and its treatment. Combined new and old treatments as well as JAK2V617F inhibitors, monitoring and comparison with CALR and its inhibitors can present novel methods for treatment of these patients. Detection of low levels of mutated JAK2V617F using sensitive and specific methods (e.g. qPCR), laboratory findings of ET patients and their comparison can contribute to MRD detection and disease monitoring during therapy.
Authorship
All authors drafted and approved this manuscript.
Acknowledgments
This work was financially supported by grant: Th94/3 from vicechancellor for Research Affairs of Ahvaz Jundishapur University of Sciences. This study is part of MSc thesis for Reza Shirzad.
Conflict of Interest
The authors declare no conflict of interest.
References
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H et al. (2011) The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 117:5019-5032. 2
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, et al. (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937-951.
- Boyd EM, Bench AJ, Goday-Fernandez A, Anand S, Vaghela KJ, et al. (2010 ) Clinical utility of routine MPL exon 10 analysis in the diagnosis of essential thrombocythaemia and primary myelofibrosis. British journal of haematology 149:250-257. 4
- Dos Santos MT, Mitne-Neto M, Miyashiro K, Chauffaille Mde L, Rizzatti EG (2014)Molecular genetic tests for JAK2V617F, Exon12_JAK2 and MPLW515K/L are highly informative in the evaluation of patients suspected to have BCR-ABL1-negative myeloproliferative neoplasms. Journal of clinical pathology67:176-178.
- Kim HJ, Jang JH, Yoo EH, Kim HJ, Ki CS, et al. (2010) JAK2 V617F and MPL W515L/K mutations in Korean patients with essential thrombocythemia. The Korean journal of laboratory medicine 30:474-476.
- Zhang SJ, Qiu HX, Li JY, Shi JY, Xu W (2010) The analysis of JAK2 and MPL mutations and JAK2 single nucleotide polymorphisms in MPN patients by MassARRAY assay. International journal of laboratory hematology 32:381-386.
- Tefferi A, Wassie EA, Guglielmelli P, Gangat N, Belachew AA, et al. (2014) Type 1 versus Type 2 calreticulin mutations in essential thrombocythemia: a collaborative study of 1027 patients. American journal of hematology89:E121-4.
- Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, et al. (2014) JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood 123:1544-1551.
- Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, et al. (2014) Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 123:1552-1555.
- Fu R, Xuan M, Zhou Y, Sun T, Bai J, et al. (2014) Analysis of calreticulin mutations in Chinese patients with essential thrombocythemia: clinical implications in diagnosis, prognosis and treatment. Leukemia 28:1912-1914.
- Andrikovics H, Krahling T, Balassa K, Halm G, Bors A, et al. (2014) Distinct clinical characteristics of myeloproliferative neoplasms with calreticulin mutations. Haematologica 99: 1184-1190
- Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, et al. (2014) Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood 123: 2220-2228
- Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100:2292-302.
- Sabattini E, Bacci F, Sagramoso C, Pileri S (2008) WHO classification of tumours of haematopoietic and lymphoid tissues in: an overview. Pathologica 102:83-87.
- Harrison CN, Bareford D, Butt N, Campbell P, Conneally E, et al. (2010) Guideline for investigation and management of adults and children presenting with a thrombocytosis. British journal of haematology 149:352-375.
- Wolanskyj AP, Lasho TL, Schwager SM, McClure RF, Wadleigh M, et al. (2005) JAK2V617F mutation in essential thrombocythaemia: clinical associations and long term prognostic relevance. British journal of haematology 131:208-213.
- Speletas M, Katodritou E, Daiou C, Mandala E, Papadakis E, et al. (2007) Correlations of JAK2-V617F mutation with clinical and laboratory findings in patients with myeloproliferative disorders. Leuk Res31:1053-1062.
- Poopak B, Hagh MF, Saki N, Elahi F, Rezvani H, et al. (2013) JAK2 V617F mutation in Iranian patients with myeloproliferative neoplasms: clinical and laboratory findings. Turkish Journal of Medical Sciences 347-353.
- Patriarca A, Pompetti F, Malizia R, Iuliani O, Di Marzio I, et al. (2010) Is the absence of JAK2 mutation a risk factor for bleeding in essential thrombocythemia? An analysis of 106 patients. Blood transfusion Trasfusione del sangue 8:21-27. 20
- Fu R, Xuan M, Lv C, Zhang L, Li H, et al. (2014) External validation and clinical evaluation of the International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in a large cohort of Chinese patients. European journal of haematology 92:502-509.
- Finazzi G, Rambaldi A, Guerini V, Carobbo A, Barbui T (2007) Risk of thrombosis in patients with essential thrombocythemia and polycythemia vera according to JAK2 V617F mutation status 135-136.
- Cho YU, Chi HS, Lee EH, Jang S, Park CJ, et al. (2009) Comparison of clinicopathologic findings according to JAK2 V617F mutation in patients with essential thrombocythemia. International journal of hematology 89:39-44. 23
- McLornan D, Percy M, McMullin MF (2006) JAK2 V617F: a single mutation in the myeloproliferative group of disorders. The Ulster medical journal 75:112-119.
- Liu PC, Caulder E, Li J, Waeltz P, Margulis A, et al. (2009) Combined inhibition of Janus kinase 1/2 for the treatment of JAK2V617F-driven neoplasms: selective effects on mutant cells and improvements in measures of disease severity. Clinical cancer research : an official journal of the American Association for Cancer Research 15:6891-6900.
- Li WX (2008) Canonical and non-canonical JAK-STAT signaling. Trends in cell biology 18:545-551. 26
- Taher A, Shammaa D, Bazarbachi A, Itani D, Zaatari G, et al. (2009) Absence of JAK2 V617F mutation in thalassemia intermedia patients. Molecular biology reports36:1555-1557.
- Zhao R, Xing S, Li Z, Fu X, Li Q, et al. (2005) Identification of an acquired JAK2 mutation in polycythemia vera. The Journal of biological chemistry 280:22788-22792. 28
- Xia J, Lu MZ, Jiang YQ, Yang GH, Zhuang Y, et al. (2012) JAK2 V617F, MPL W515L and JAK2 Exon 12 Mutations in Chinese Patients with Primary Myelofibrosis. Chinese journal of cancer research Chung-kuo yen cheng yen chiu 24:72-76.
- Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, et al. (2014) CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia 28:1472-1477. 30
- Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, et al. (2014) Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 124:1062-1069. 31
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, et al. (2013) Somatic mutations of calreticulin in myeloproliferative neoplasms. The New England journal of medicine. 369:2379-2390.
- Jovanovic JV, Ivey A, Vannucchi AM, Lippert E, Oppliger Leibundgut E, et al. (2013) Establishing optimal quantitative-polymerase chain reaction assays for routine diagnosis and tracking of minimal residual disease in JAK2-V617F-associated myeloproliferative neoplasms: a joint European LeukemiaNet/MPN&MPNr-EuroNet (COST action BM0902) study. Leukemia 27:2032-2039.
- Campbell PJ, Bareford D, Erber WN, Wilkins BS, Wright P, et al. (2009) Reticulin accumulation in essential thrombocythemia: prognostic significance and relationship to therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27:2991-2999. 34
- Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, et al. (2009) A randomized trial of rosuvastatin in the prevention of venous thromboembolism. The New England journal of medicine360:1851-1861.
- Lee D, Oka T, Hunter B, Robinson A, Papp S, et al. (2013) Correction: Calreticulin Induces Dilated Cardiomyopathy. PloS one. 36
- Wang W-A, Groenendyk J, Michalak M (2012 ) Calreticulin signaling in health and disease. The international journal of biochemistry & cell biology 44:842-846.
- Da Silva RR, Domingues Hatzlhofer BL, Machado CG, Lima AS, de Albuquerque DM, et al. (2012) JAK2 V617F mutation prevalence in myeloproliferative neoplasms in Pernambuco, Brazil. Genetic testing and molecular biomarkers. 16:802-805.38
- Reuther GW(2008) JAK2 activation in myeloproliferative neoplasms: a potential role for heterodimeric receptors. Cell cycle 7:714-719.
- Lippert E, Girodon F, Hammond E, Jelinek J, Reading NS, , et al (2009) Concordance of assays designed for the quantification of JAK2V617F: a multicenter study. Haematologica 94:38-45. 40
- Bench AJ, White HE, Foroni L, Godfrey AL, Gerrard G, et al. (2013) Molecular diagnosis of the myeloproliferative neoplasms: UK guidelines for the detection of JAK2 V617F and other relevant mutations. British journal of haematology160:25-34.
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, et al. (2013) Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. The New England journal of medicine 369:2391-405.
- Nakamura K, Zuppini A, Arnaudeau S, Lynch J, Ahsan I, et al. (2001) Functional specialization of calreticulin domains. The Journal of cell biology 154:961-972. 43
- Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N (2013) Calreticulin in the immune system: ins and outs. Trends in immunology34:13-21.
- Bibi A, Agarwal NK, Dihazi GH, Eltoweissy M, Van Nguyen P, et al. (2011) Calreticulin is crucial for calcium homeostasis mediated adaptation and survival of thick ascending limb of Henle's loop cells under osmotic stress. The international journal of biochemistry & cell biology 43:1187-1197. 45
- Szabo E, Qiu Y, Baksh S, Michalak M, Opas M(2008) Calreticulin inhibits commitment to adipocyte differentiation. The Journal of cell biology 182:103-116.
- Maffioli M, Genoni A, Caramazza D, Mora B, Bussini A, et al. (2014) Looking for CALR mutations in familial myeloproliferative neoplasms. Leukemia 28:1357-1360. 47
- Green D (2009) Risk of future arterial cardiovascular events in patients with idiopathic venous thromboembolism. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 259-266.
- Barbui T, Carobbio A, Rambaldi A, Finazzi G (2009) Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood 114:759-763. 49
- Sozer S, Fiel MI, Schiano T, Xu M, Mascarenhas J, et al. (2009) The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 113:5246-5249.
- Antonioli E, Guglielmelli P, Poli G, Bogani C, Pancrazzi A, et al. (2008) Influence of JAK2V617F allele burden on phenotype in essential thrombocythemia. Haematologica 93:41-48. 51
- Vannucchi AM, Antonioli E, Guglielmelli P, Pancrazzi A, Guerini V, et al. (2008 ) Characteristics and clinical correlates of MPL 515W>L/K mutation in essential thrombocythemia. Blood 112:844-847.
- Regina S, Herault O, D'Alteroche L, Binet C, Gruel Y(2007) JAK2 V617F is specifically associated with idiopathic splanchnic vein thrombosis. Journal of thrombosis and haemostasis : JTH 5:859-861. 53
- Rossi D, Cresta S, Destro T, Vendramin C, Bocchetta S, et al. (2007) JAK2V617F in idiopathic venous thromboembolism occurring in the absence of inherited or acquired thrombophilia. British journal of haematology 138:813-814.
- Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, et al. (2007) Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 110:840-846.55
- Gangat N, Wassie EA, Lasho TL, Finke C, Ketterling RP, et al. (2014) Mutations and thrombosis in essential thrombocythemia: prognostic interaction with age and thrombosis history. European journal of haematology.
- Campbell PJ, Griesshammer M, Dohner K, Dohner H, Kusec R, et al. (2006) V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood 107:2098-2100. 57
- Rumi E, Pietra D, Guglielmelli P, Bordoni R, Casetti I, et al. (2013) Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 121:4388-4395.
- Kaufman RJ, Pipe SW, Tagliavacca L, Swaroop M, Moussalli M(1997) Biosynthesis, assembly and secretion of coagulation factor VIII. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis 2:S3-14.59
- Kuwabara K, Pinsky DJ, Schmidt AM, Benedict C, Brett J, et al. (1995) Calreticulin, an antithrombotic agent which binds to vitamin K-dependent coagulation factors, stimulates endothelial nitric oxide production, and limits thrombosis in canine coronary arteries. The Journal of biological chemistry 270:8179-8187.
- Ding H, Hong C, Wang Y, Liu J, Zhang N, Shen C, et al. (2014) Calreticulin promotes angiogenesis via activating nitric oxide signalling pathway in rheumatoid arthritis. Clinical and experimental immunology. 178:236-244. 61
- Dai E, Stewart M, Ritchie B, Mesaeli N, Raha S, et al. (1997) Calreticulin, a potential vascular regulatory protein, reduces intimal hyperplasia after arterial injury. Arteriosclerosis, thrombosis, and vascular biology 17:2359-2368.
- Pinsky DJ, Kuwabara K, Schmidt AM, Lawson CA, Benedict C, et al. (1996) Calreticulin and Thrombosis. Calreticulin: Springer 155-168.
- Stern DM, Kuwabara K, Benedict C, Ryan J(1995) Calreticulin: a novel antithrombotic agent. Google Patents.
- Griesshammer M, Bangerter M, van Vliet HH, Michiels JJ (1997) Aspirin in essential thrombocythemia: status quo and quo vadis. Seminars in thrombosis and hemostasis 23:371-377.
- Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, et al. (2004) Efficacy and Safety of Low-Dose Aspirin in Polycythemia Vera. New England Journal of Medicine 350:114-124.
- Alvarez-Larran A, Cervantes F, Pereira A, Arellano-Rodrigo E, Perez-Andreu V, et al. (2010 ) Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood 116:1205-121067.
- Reikvam H, Tiu RV (2012) Venous thromboembolism in patients with essential thrombocythemia and polycythemia vera. Leukemia 26:563-571.
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, et al. (1995) Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. The New England journal of medicine 332:1132-1136.
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, et al. (2005) Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. The New England journal of medicine 353:33-45. 70
- Baskin R, Majumder A, Sayeski PP (2010) The recent medicinal chemistry development of Jak2 tyrosine kinase small molecule inhibitors. Current medicinal chemistry 17:4551-4558.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences